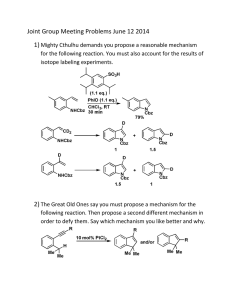
Block 3, Workshop 2 Adam Nelson a.s.nelson@leeds.ac.uk 1. Propose approaches that would be suitable for the parallel synthesis of analogues In each case, the group shown in blue must be variable. You should consider which reactant classes will likely enable this variation 1. Propose approaches that would be suitable for the parallel synthesis of analogues 1. Propose approaches that would be suitable for the parallel synthesis of analogues 2. This question concerns the synthesis of a potential anticancer drug (a) How would you remove (separately) the protecting groups in the starting material? (b) Propose a synthesis of the spirocyclic intermediate (c) Propose how the spirocyclic intermediate might be decorated to give the illustrated analogue 2. This question concerns the synthesis of a potential anticancer drug (a) How would you remove (separately) the protecting groups in the starting material? Boc (TFA) and Cbz (H2, Pd/C) 3. This question concerns the synthesis of a drug 1. Propose reactants and reagents for Step 1 2. Propose a mechanism for Step 2 3. Propose a mechanism for Step 3 and rationalise the regioselectivity 3. This question concerns the synthesis of a drug