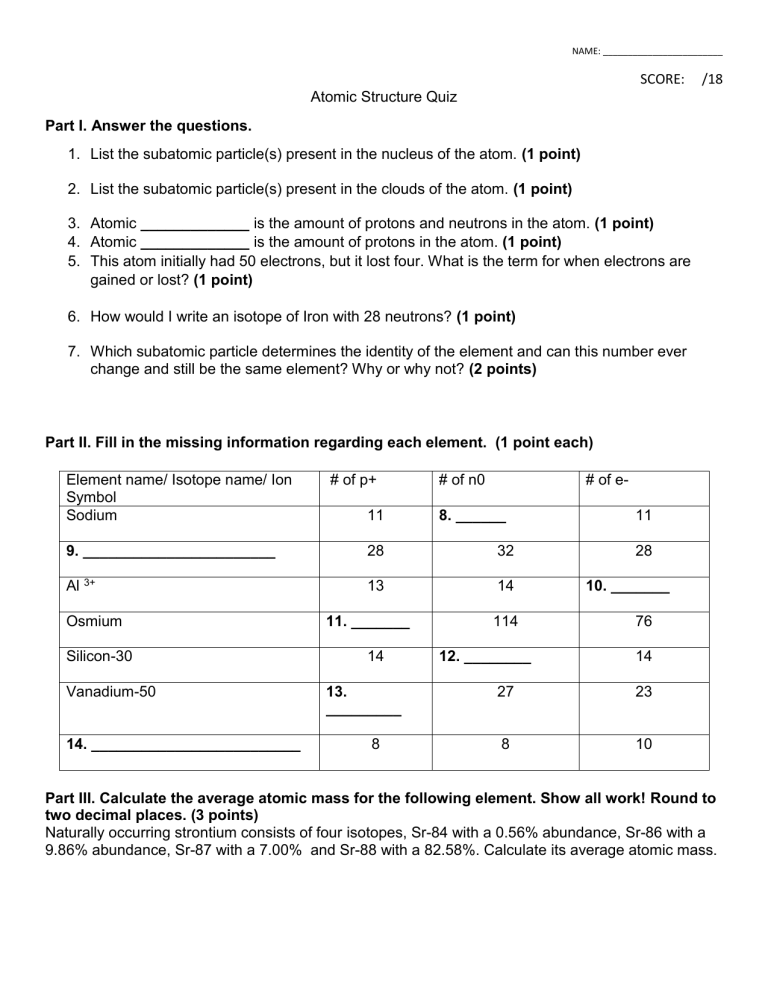
NAME: ________________________ SCORE: /18 Atomic Structure Quiz Part I. Answer the questions. 1. List the subatomic particle(s) present in the nucleus of the atom. (1 point) 2. List the subatomic particle(s) present in the clouds of the atom. (1 point) 3. Atomic _____________ is the amount of protons and neutrons in the atom. (1 point) 4. Atomic _____________ is the amount of protons in the atom. (1 point) 5. This atom initially had 50 electrons, but it lost four. What is the term for when electrons are gained or lost? (1 point) 6. How would I write an isotope of Iron with 28 neutrons? (1 point) 7. Which subatomic particle determines the identity of the element and can this number ever change and still be the same element? Why or why not? (2 points) Part II. Fill in the missing information regarding each element. (1 point each) Element name/ Isotope name/ Ion Symbol Sodium # of p+ 11 # of n0 # of e- 8. ______ 9. _______________________ 28 32 Al 3+ 13 14 Osmium Silicon-30 Vanadium-50 14. _________________________ 11. _______ 14 13. _________ 8 114 12. ________ 11 28 10. _______ 76 14 27 23 8 10 Part III. Calculate the average atomic mass for the following element. Show all work! Round to two decimal places. (3 points) Naturally occurring strontium consists of four isotopes, Sr-84 with a 0.56% abundance, Sr-86 with a 9.86% abundance, Sr-87 with a 7.00% and Sr-88 with a 82.58%. Calculate its average atomic mass.


