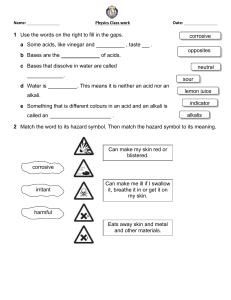
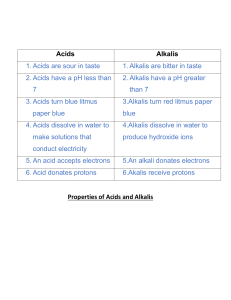
Textile Fiber Reactions: Acids, Alkalis, Oxidizing Agents
advertisement
KYAMBOGO UNIVERSITY FACULTY OF SCIENCE DEPARTMENT OF CHEMISTRY BACHELOR OF SCIENCE TECHNOLOGY CHEMISTRY COURSE UNIT: SST 3201: TEXTILE TECHNOLOGY NAME: LUMANYIKA EDRINE REG NO: 20/U/CTD/11286/GV LECTURER: MR. AKAMPURIRA DENIS TASK: INDIVIDUAL ASSIGNMENT SIGNATURE: ………………………………... Question Describe the reactions of different fibres/polymers with; acids, alkalis and oxidizing agents. A fibre is defined as a small threadlike structure (1). The American Society for Testing and Materials (ASTM) defines a ‘fibre’ as ‘a generic term for any one of the various types of matter that form the basic element of a textile, and it is characterised by having a length at least 100 times its diameter’ (2). The Textile Institute defines a fibre as a ‘textile raw material, generally characterised by flexibility, fineness and high ratio of length to thickness’ (3). A similar industry definition is a ‘unit matter with a length. A fibre is a flexible material, generally characterized by good flexibility, fineness and high ratio of length to thickness. The practical understanding of the word ‘fibre’ is that it is a unit of cylindrical matter characterized by a reasonably long length and small diameter. Fibres are strands of long and flexible materials (hair-like), which are either natural or manufactured and form the basic elements of fabrics and other textiles. In a broad sense, the word ‘fibre’ is used for all the types of matter – either natural or manmade, forming the basic structural elements of any textile fabrics and other types of textile structures. Natural fibre can be termed as a fine strand of material which can be sourced from the parts of plants/animal. Cellulosic fibres Reaction with Acids Acids are the most destructive agents for cellulose, attacking the glycosidic linkages by an acid-catalysed hydrolysis reaction. The initial rapid increase in fluidity arises from the ease of attack of the acid in the more amorphous, readily accessible regions of the fibre. The rate of reaction slows down as the reaction then occurs at the chain ends of the more inaccessible regions. The acid hydrolysis reaction causes the formation of both reducing and non-reducing groups at the ends of the polymer chains. Reaction with Alkali The situation with alkali is very different from that with acid, so that whilst cotton fibres are readily degraded by acid, they are much more stable in alkali. However, that is not to say the alkali has no effect on cotton fibres and, at high temperatures especially, alkali can also cause hydrolysis of the cellulose. In this case, the reaction occurs in a stepwise manner by the removal of anhydro glucose units from the reducing ends of the cellulosic chains. Whilst the rate of the reaction is slow, there is nevertheless an important consequence for the processing of cotton materials, because in the scouring of cotton to remove impurities, such as fats and waxes from the fibres, alkaline conditions (soap and soda) at the boil are used. The most important action of alkali on cotton is the swelling of the fibres. Reaction with Oxidizing agents. The glucosidic repeating unit structure of cellulose contains three hydroxyl groups: one primary and two secondary groups. As with all alcohols, these groups can be oxidised: the primary alcohols are oxidised firstly to an aldehyde and then to a carboxylic acid, and the secondary alcohols are oxidised to the corresponding ketone. In addition, it is possible that cleavage of the glucosidic ring can occur, both between the C-2 and C-3 carbon atoms and between the C-1 and the oxygen atom of the ring. The products of oxidation are called ‘oxycelluloses’, though they can vary in their chemical nature quite considerably. A wide variety of oxidising agents can cause such degradation, with hydrogen peroxide, sodium chlorite and sodium hypochlorite being. Wool fibre Reaction with Acid. Wool fibres are more resistant to acids. Indeed, they are often dyed under acidic conditions, with some types of dye being applied at a pH as low as 2. Dyes applied to wool are anionic and therefore at lower pH there is extensive ionic attraction between dye and fibres. Under much harsher conditions, acids hydrolyse the peptide linkages in keratins to their constituent amino acids, with the exception of tryptophan, which is normally destroyed. Reaction with Alkali. Wool is particularly prone to damage by alkali. Indeed, it can be dissolved completely in 5% sodium hydroxide solution at 100 °C. Alkali attacks the disulfide bridges and can hydrolyse the peptide linkages along each keratin chain. Thus, the use of alkali in wool processing technology has to be severely restricted. The chief products formed from attack by alkali are lanthionine and lysinoalanine residues. Minor products, such a β-aminoalanine and ornithinoalanine residues, have also been isolated. The initial steps appear to involve deprotonation of β-carbon atoms in disulfide bridges and the formation of dehydroalanine residues. Cystine residues and sulfur are also produced. Reactions with oxidizing agents Reduction of wool is important technologically, as it forms the basis of the setting of creases and pleats in wool fabrics. The reagents most commonly used to reduce wool are thiols and phosphines. Amongst all the amino acid residues, only the cystine residues are affected. For complete reduction, therefore, a large excess of thiol is needed. Among the thiols that have been applied are thioglycolic acid (HS-CH2-COOH), its ammonium salt and toluene-ω-thiol (C6H5-CH2-SH). Polyester fibre Reaction to Acids Weak acids, even at the boiling point, have no effect on polyester fibres unless the fibres are exposed for several days. Polyester fibres have good resistance to strong acids at room temperature. Prolonged exposure to boiling hydrochloric acid destroys the fibres, and 96% sulfuric acid and causes disintegration of the fibres. Polyester is resistant to mineral and organic acids. Highly concentrated acids at high temperatures cause degradation such as sulphuric acid. Reaction to Alkali At room temperature, polyester has good resistance to weak alkalis and fair resistance to strong alkalis. It reduces with increase in temperature and alkalis concentration. It exhibits only moderate resistance to strong alkalis at room temperature and is degraded at elevated temperatures. Action of oxidizing agents Fabrics of polyester may be safely bleached, because polyesters have good resistance to deterioration to household bleaches. If the polyester has optical brightener, bleaching is not necessary. Silk fibre Reaction with Acid and Alkali. Treatment of silk with acid or alkali gives rise to hydrolysis of the fibroin chains. The degree of hydrolysis is highly dependent on pH. Hydrolysis is greater under acidic conditions than in alkaline conditions, but hot concentrated acids and alkalis both readily decompose silk. The lowest degree of hydrolysis occurs in the pH range of 4–8. The peptide bonds that are primarily attacked are those adjacent to serine and threonine residues. These ester groups are more prone to hydrolysis than the peptide bonds from which they originate. Sulfuric acid also sulfonates the tyrosine residues in the fibroin chains. Both acids and alkalis, under carefully controlled conditions, can induce a crepe effect, in which silk fabrics have a slightly wrinkled appearance. Such an appearance can be aesthetically desirable. Dilute organic acids, such as ethanoic, tartaric and citric acids, can enhance a rustling effect produced in silk fabrics, known as ‘scroop’. Reactions with oxidizing agents Oxidising agents, such as hydrogen peroxide, performic acid and peracetic acid, induce several different types of reaction. Peptide bonds, located at tyrosine residues in the fibroin chains, are broken, and amino groups at the ends of the chains can also be oxidised. Moreover, tyrosine residues themselves may be oxidised by potassium permanganate and chlorinating agents. 1,4-benzoquinone is released as a result of oxidation, and cross-linking is thought then to occur between free amino groups, such as those in lysine residues. Viscose rayons fibre Viscose rayons fibre is made from wood pulp. It is also known as artificial silk as it has characteristics resembling silk. Rayon is mainly used in clothing, carpets, medical dressings and for insulation. Reaction with Acids Being pure cellulose, the fabric is disintegrated by hot dilute and cold concentrated acids similar to that of cotton. Reaction with Alkali Concentrated solutions of alkalis disintegrate viscose rayon. A mild soap with lukewarm water is recommended in washing rayons. Reaction with oxidizing agents Household bleaches containing sodium hypochlorite, sodium perborate or hydrogen peroxide may be safely used. Acrylic Fibres Reaction with Acid and Alkali. Acrylic fibres have high resistance to mineral acids and to weak alkalis, but strong alkalis cause rapid degradation, especially at elevated temperatures. For example, immersion of acrylic fibres in 5% sodium hydroxide at 80 °C for 1 day will cause complete degradation, whilst immersion for 1 day at 75 °C in 60% sulfuric acid causes only slight degradation. Reactions with oxidizing agents Oxidising agents have little effect on acrylic fibres, especially in comparison with other fibre types. For example, it has been shown that exposure of the acrylic fibre Orlon to bleaching fluid for up to 9 hours reduces its tenacity by about 9%, whilst under similar conditions the tenacity of nylon is reduced by 50% and cotton is almost totally destroyed. REFERENCES Kudlacek L, Hepnen M, Kasparova Z (1986): Morphology of Polymers. Ed by B. Sedlacek, Walter de Gruyter and Co, Berlin, New York. Hinrichsen G, Iburg A, Eberhardt A, Springer H, Wolbring P (1979): Colloid and Polymer Science, 257:1251. Robert R. Mather and Roger H. Wardman (2015). The Chemistry of Textile Fibres 2nd Edition. The Royal Society of Chemistry, Thomas Graham House, Science Park, Milton Road, Cambridge CB4 0WF, UK.