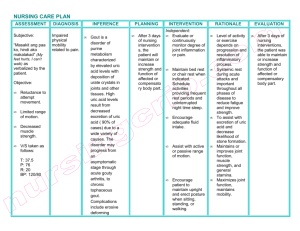
James Ivan A. Baluran Pharmacology Laboratory (A1) Clinical Case: Gout Group 1 Clinical Questions: 1. What is gout? ● Gout is an inflammatory disease in which MSU crystals deposit into a joint, making it red, tender and swollen within hours. When this happens, it is called a Gouty Attack. The underlying cause of gout is hyperuricemia, which results in sharp needle-like crystals in areas with slow blood flow like the joints and the kidney tubules. Overtime repeated gouty attacks can cause destruction of the joint tissue which results in arthritis. 2. What are the risk factors for gout? ● Male ● Alcohol consumption ● Hypertension ● Obesity ● Diabetes ● Dyslipidemia ● Hyperuricemia due to underexcretion or overproduction 3. What are the four clinical phases of gout? a. Asymptomatic ● Hyperuricemia with no symptoms ● May last ≥ 10 years b. Acute Gouty Arthritis ● Acute severe pain with overlying erythema, decreased range of motion, swelling, warmth ● Possibly fever ● Symptoms are more likely to occur at night, typically waking the patient. ● Symptoms peak after 12–24 hours and regress over days to weeks. ● Desquamation of the skin overlying the joint may be seen during the recovery from an acute gout flare. c. Intercritical stage ● Asymptomatic ● May last up to several years d. Chronic Gouty Arthritis ● Progressive joint destruction ● Tophi formation a. Multiple painless hard nodules with possible joint deformities ○ May appear yellow or white because of overlying attenuated skin ○ Ulceration and discharge (chalky white substance) may occur ● b. Bone tophi: urate crystal deposition in bones (e.g., elbows, knees, extensor surfaces of forearms) c. Soft tissue tophi: urate crystal deposition in the pinna of the external ear, subcutis, tendon sheaths (e.g., at the Achilles tendon), or synovial bursae (e.g., olecranon bursa) Renal manifestations with uric acid nephrolithiasis and uric acid nephropathy 4. What are the diagnostic criteria for gout? Diagnostic criteria for gout: ● Male sex. ● Previous arthritis attack. ● Onset within 1 day. ● Joint redness. ● First metatarsophalangeal joint involvement. ● Hypertension or 1 or more cardiovascular diseases. ● A serum uric acid level higher than 5.88 mg/dL. 5. What are the workup approach considerations for gout? The workup approach consideration for gout are : a)Synovial Fluid AnalysisWhen a patient presents with acute inflammatory monoarticular arthritis, aspiration of the involved joint is critical to rule out an infectious arthritis and to attempt to confirm a diagnosis of gout or pseudogout on the basis of identification of crystals. b)Serum Uric AcidMeasurement of serum uric acid is the most misused test in the diagnosis of gout. The presence of hyperuricemia in the absence of symptoms is not diagnostic of gout. In addition, as many as 15% of patients with symptoms from gout may have normal serum uric acid levels at the time of their attack. Thus, the diagnosis of gout can be missed if the joint is not aspirated. Approximately 25% of the population has a history of elevated serum uric acid, but only a minority of patients with hyperuricemia develop gout. Thus, an abnormally high serum uric acid level does not indicate or predict gout. As noted, gout is diagnosed by the presence of urate crystals in the synovial fluid or soft tissues. More importantly, some patients who present with a hot swollen joint and an elevated serum uric acid level in fact have infectious arthritis, which may be mismanaged if their synovial fluid is not examined. c)Urinary Uric Acid A 24-hour urinary uric acid evaluation is generally performed if uricosuric therapy is being considered. If patients excrete more than 800 mg of uric acid in 24 hours while eating a regular diet, they are over excretors and thus overproducers of uric acid. d)Blood studies Blood studies may reveal abnormalities associated with gout or common comorbid conditions.The WBC count may be elevated in patients during the acute gouty attack, particularly if it is polyarticular. Hypertriglyceridemia and low levels of high-density lipoprotein (HDL) are associated with gout. Glucose measurement is useful because patients with gout are at increased risk for the development of diabetes mellitus. e)Radiography Plain radiographs may show findings consistent with gout.Erosions with overhanging edges generally are considered pathognomonic for gout. f)Ultrasonography Ultrasonographic findings in established gout include the following : -A “double-contour” sign, consisting of a hyperechoic, irregular line of MSU crystals on the surface of articular cartilage overlying an adjacent hyperechoic bony contour -“Wet clumps of sugar,” representing tophaceous material, described as hyperechoic and hypoechoic heterogeneous material with an anechoic rim -Bony erosions adjacent to tophaceous deposits g)Histology Chronic tophaceous gouty deposits frequently show large pale pink acellular areas, which represent dissolved urate crystals, surrounded by histiocytes and multinucleated giant cells. Pharmacology Guide Questions: 1. How is gout managed? Gout is managed in the following 3 stages 1. Treating the acute attack 2. Providing prophylaxis to prevent acute flares 3. Lowering excess stores of urate to prevent flares of gouty arthritis and to prevent tissue deposition of urate crystals The American College of Rheumatology (ACR) published guidelines on the treatment and prophylaxis of acute gouty arthritis and the management of hyperuricemia.While those guidelines do describe treatment targets, more recent publications have focused more closely on the treat-to-target concept, although for the most part these recommendations are based on underlying principles and expert opinion rather than trial data. As a general rule, asymptomatic hyperuricemia should not be treated, though ultrasonographic studies have demonstrated that urate crystal deposition into soft tissues occurs in a minority of patients with asymptomatic hyperuricemia.Patients with levels higher than 11 mg/dL who over excrete uric acid are at risk for renal stones and renal impairment; therefore, renal function should be monitored in these individuals. Urate-lowering therapy appears to reduce the incidence of kidney damage in gout. In a retrospective study of 16,186 patients with initial serum uric acid levels above 7 mg/dL, Levy and colleagues found that patients with gout who remained on urate-lowering therapy were less likely to develop kidney damage leading to chronic kidney disease than those who were untreated. All patients were followed for 36 months from their first documented high serum uric acid level. Patients 65 years of age and older were more likely to have three or more flares. Other risk factors for gout flares included the following: 1. 2. 3. 4. 5. 6. Male gender Failure to attain serum uric acid goal Presence of three or more comorbidities Use of diuretics No changes in initial urate-lowering therapy dose Nonadherence to urate-lowering therapy Tophi should not be surgically removed unless they are in a critical location or drain chronically. Surgery may be indicated for tophaceous complications, including infection, joint deformity, compression (eg, cauda equina or spinal cord impingement), and intractable pain, as well as for ulcers related to tophaceous erosions. Delayed healing is noted in 50% of patients. 2. Current treatment guidelines a. Nonsteroidal Anti-inflammatory Drugs (NSAIDs) i. Aspirin 1. Aspirin is a salicylate that exhibits analgesic, anti-inflammatory, and antipyretic activities. Additionally, it also inhibits platelet aggregation. a. Indication: Rheumatic disorders, Fever, Mild and Moderate pain b. Mechanism of Action: It is a selective and irreversible inhibitor of cyclooxygenase-1 (COX-1) enzyme resulting in direct inhibition of the biosynthesis of prostaglandins and thromboxanes from arachidonic acid. c. Dosage: Adult: 4-8 g daily in divided doses for acute i. disorders. 5.4 g daily in divided doses for chronic conditions. d. Adverse Effects: i. Significant: Salicylate sensitivity, tinnitus. Rarely, Reye’s syndrome. Hypersensitivity ii. reactions (e.g. Stevens Johnson syndrome, angioedema), gastrointestinal bleeding and perforation. ii. iii. Other non-selective COX inhibitors (Ibuprofen, Naproxen, Indomethacin, Ketorolac) 1. Nonselective NSAIDs vary primarily in their potency, analgesic and anti-inflammatory effectiveness, and duration of action. Ibuprofen and naproxen have moderate effectiveness; indomethacin has greater anti-inflammatory effectiveness; and ketorolac has greater analgesic effectiveness. a. Indication: Rheumatic disorders, mild to sever pain b. Mechanism of action: Inhibit both cyclooxygenase isoforms and thereby decrease prostaglandin and thromboxane synthesis throughout the body. Release of prostaglandins necessary for homeostatic function is disrupted, as is release of prostaglandins involved in inflammation. c. Dosage: i. Naproxen: 1. 500 mg BID ii. Indomethacin: 25-50 mg TID iii. Ketorolac: Maximum dose: 40 mg/day iv. Ibuprofen: 800 mg BID v. Diclofenac: 50 mg TID d. Adverse effects i. Most prominent: GI irritations, gastrointestinal and renal damage, Selective COX-2 inhibitors (Celecoxib, Etoricoxib, Valdecoxib) 1. nonsteroidal anti-inflammatory drug (NSAID). It works by reducing hormones that cause inflammation and pain in the body. The COX-2-selective inhibitors have less effect on the prostaglandins involved in homeostatic function, particularly those in the gastrointestinal tract a. Indication: Rheumatoid arthritis, gout, menstrual pain b. Mechanism of action: selectively inhibits cyclo-oxygenase-2 activity (COX-2), reducing hormones that cause inflammation and pain in the body. c. Dosage: i. Celecoxib: 800 mg followed by 400 mg 12 hours later, then 400 mg BID ii. Etoricoxib:The normal dosage for acute gout is 120 mg once a day for up to eight days. d. Adverse effects: stomach or intestinal bleeding, fatal. Renal damage, myocardial infarction and stroke (rofecoxib and valdecoxib) b. Uricosuric agents Drugs employed to decrease the body pool of urate in patients with tophaceous gout or in those with increasingly frequent gout attacks. In a patient who secretes large amounts of uric acid, the uricosuric agents should not be used. Probenecid & Sulfinpyrazone a. Indication: Patients with underexcretion of uric acid when allopurinol or febuxostat is contraindicated or when tophi are present. Therapy should not be started until 2–3 weeks after an acute attack. b. Mechanism of Action: Inhibit active transport sites for reabsorption and secretion in the proximal renal tubule so that net reabsorption of uric acid in the proximal tubule is decreased. c. Dosage: i. Probenecid started at a dosage of 0.5g orally in divided doses (progress to 1g daily after 1 week. ii. Sulfinpyrazone started at a dosage of 200 mg orally daily, (progress to 400-800 mg daily in divided doses d. Adverse Effects: GI irritation, rash, nephrotic syndrome (Probenecid), and both may rarely cause aplastic anemia c. Corticosteroids Corticosteroids are man-made drugs that closely resemble cortisol, a hormone that your adrenal glands produce naturally. Corticosteroids are often referred to by the shortened term "steroids.” Corticosteroids (cortisone-like medicines) are used to provide relief for inflamed areas of the body. They lessen swelling, redness, itching, and allergic reactions. a. Indication: rheumatoid arthritis, inflammatory bowel disease (IBD), asthma, allergies and many other conditions. b. Mechanism of action: Corticosteroids enter the cell and bind to cytosolic receptors that transport the steroid into the nucleus. The steroid-receptor complex alters gene expression by binding to glucocorticoid response elements (GREs) or mineralocorticoid-specific elements .Tissue-specific responses to steroids are made possible by the presence in each tissue of different protein regulators that control the interaction between the hormone-receptor complex and particular response elements. c. Dosage: Oral: 10 to 60 mg/day given in a single daily dose or in 2 to 4 divided doses; Low dose: 2.5 to 10 mg/day; High dose: 1 to 1.5 mg/kg/day (usually not to exceed 80 to 100 mg/day). Glucocorticoid-Responsive Conditions: 5-60 mg/day PO in single daily dose or divided q6-12h d. Adverse Effect: weight gain or swelling of the legs (edema),High blood pressure, Loss of potassium, Headache,Muscle weakness, Puffiness of the face (moon face), Facial hair growth,Slow wound healing, Glaucoma, Cataracts, Ulcers in the stomach and duodenum,Loss of diabetes control d. Xanthine Oxidase Inhibitors i. Allopurinol- preferred and standard-of-care therapy for gout during the period between acute episodes. a. Indications: Allopurinol is often the first-line agent for the treatment of chronic gout in the period between attacks and it tends to prolong the intercritical period. b. Mechanism of Action: Xanthine oxidase inhibitor; inhibits conversion of hypoxanthine to xanthine to uric acid; decreases production of uric acid without disrupting synthesis of vital purines. c. Dosage: The initial dosage of allopurinol is 50–100 mg/d. d. Adverse Effects: In addition to precipitating gout (the reason to use concomitant colchicine or NSAID), GI intolerance (including nausea, vomiting, and diarrhea), peripheral neuritis and necrotizing vasculitis, bone marrow suppression, and aplas- tic anemia may rarely occur. II. Febuxostat- is a potent and selective inhibitor of xanthine oxidase, thereby reducing the formation of xanthine and uric acid without affecting other enzymes in the purine or pyrimidine metabolic pathway. A. Indications: Febuxostat is approved at doses of 40 or 80 mg for the treatment of chronic hyperuricemia in gout patients. B. Mechanism of Action: Xanthine oxidase inhibitor; inhibits conversion of hypoxanthine to xanthine to uric acid; at therapeutic dosages, decreases production of uric acid without disrupting synthesis of vital purines and pyrimidines. C. Dosage: The recommended starting dose of febuxostat is 40 mg daily. D. Adverse Effect:Gout flares, xanthine deposition, Increased serum AST/ALT, increased TSH e. Selective Uric Acid Reabsorption Inhibitor (SURI) i. Brand names: Duzallo, Lesinurad, Zurampic a. Indication: Selective uric acid reabsorption inhibitor (SURI) is a class of medication prescribed for excessive uric acid in the blood (hyperuricemia) associated with gout. b. Mechanism of Action: Selective uric acid reabsorption inhibitor (SURI) inhibits uric acid transporter 1 (URAT1) and organic anion transporter 4 (OAT4), increasing urinary excretion of uric acid, lowering plasma urate concentrations, and eventually reducing urate deposits in the tissue. c. Dosage: 200 mg once daily. Should be taken with food. Take in the morning. d. Adverse effects: i. Significant: Gout flares, increased serum creatinine, renal failure, nephrolithiasis. ii. Gastrointestinal disorders: Gastroesophageal reflux disease. iii. Immune system disorders: Rarely, hypersensitivity reactions (e.g. photosensitivity). iv. Infections and infestations: Influenza. v. Nervous system disorders: Headache. f. Rheumatologics i. DMARDs: RA is a progressive immunologic disease that causes significant systemic effects, shortens life, and reduces mobility and quality of life. The conventional synthetic agents include small molecule drugs such as methotrexate, azathioprine, chloroquine and hydroxychloroquine, cyclophosphamide, cyclosporine, leflunomide, mycophenolate mofetil and sulfasalazine. ii. Methotrexate: Methotrexate, a synthetic nonbiologic antimetabolite, is the first-line csDMARD for treating RA and is used in 50–70% of patients. It is active in this condition at much lower doses than those needed in cancer chemotherapy. Dosage and Indications: It is recommended to start treatment with 7.5 mg weekly. According to patient response, methotrexate is increased to the most common dosing regimen for the treatment of RA, which is 15–25 mg weekly. Notably there is an increased effect up to 30–35 mg weekly, although with increased toxicity. The drug decreases the rate of appearance of new erosions. Evidence supports its use in juvenile chronic arthritis, and it has been used in psoriasis, PA, AS, polymyositis, dermatomyositis, Wegener’s granulomatosis, giant cell arteritis, SLE, and vasculitis. Mechanism of Action: Methotrexate’s principal mechanism of action at the low doses used in rheumatic diseases probably relates to inhibition of amino-imidazolecarboxamide ribonucleotide (AICAR) transformylase and thymidylate synthetase. AICAR, which accumulates intracellularly, competitively inhibits AMP deaminase, leading to an accumulation of AMP. The AMP is released and converted extracellularly to adenosine, which is a potent inhibitor of inflammation. As a result, the inflammatory functions of neutrophils, macrophages, dendritic cells, and lymphocytes are suppressed. Methotrexate has direct inhibitory effects on proliferation and stimulates apoptosis in immune inflammatory cells. Additionally, it inhibits proinflammatory cytokines linked to rheumatoid synovitis. Adverse Effect: Nausea and mucosal ulcers are the most common toxicities. Additionally, many other side effects such as leukopenia, anemia, stomatitis, GI ulcerations, and alopecia are probably the result of inhibiting cellular proliferation. Sulfasalazine Mechanism of Action: Sulfasalazine, a csDMARD, is metabolized to sulfapyridine and 5-aminosalicylic acid. The sulfapyridine is probably the active moiety when treating RA (unlike inflammatory bowel disease. Some authorities believe that the parent compound, sulfasalazine, also has an effect. Suppression of T-cell responses to concanavalin and inhibition of in vitro B-cell proliferation are documented. In vitro, sulfasalazine or its metabolites inhibit the release of inflammatory cytokines produced by monocytes or macrophages—eg, IL-1, -6, and -12, and TNF-α. Indications and Dosage: Sulfasalazine is effective in RA and reduces radiologic disease progression. It has also been used in juvenile chronic arthritis, PsA, inflammatory bowel disease, AS, and spondyloarthropathy-associated uveitis. The usual regimen is 2–3 g/d. Adverse Effect: Approximately 30% of patients using sulfasalazine discontinue the drug because of toxicity. Common adverse effects include nausea, vomiting, headache, and rash. Hemolytic anemia and methemoglobinemia also occur, but rarely. Neutropenia occurs in 1–5% of patients, while thrombocytopenia is very rare. Pulmonary toxicity and positive double-stranded DNA (dsDNA) are occasionally seen, but drug-induced lupus is rare. Reversible infertility occurs in men, but sulfasalazine does not affect fertility in women. The drug does not appear to be teratogenic Hydroxychloroquine Mechanism of Action: Chloroquine and hydroxychloroquine are non biologic drugs mainly used for malaria and in rheumatic diseases as csDMARDs. The following mechanisms have been proposed: suppression of T-lymphocyte responses to mitogens, inhibition of leukocyte chemotaxis, stabilization of lysosomal enzymes, processing through the Fc-receptor, inhibition of DNA and RNA synthesis, and the trapping of free radicals. Indications and Dosage: Antimalarials are approved for RA, but they are not considered very effective DMARDs. Dose-loading may increase the rate of response. There is no evidence that these compounds alter bony damage in RA at their usual dosages (up to 6.4 mg/kg per day for hydroxychloroquine or 200 mg/d for chloroquine). It usually takes 3–6 months to obtain a response. Antimalarials are used very commonly in SLE because they decrease mortality and the skin manifestations, serositis, and joint pains of this disease. They have also been used in Sjögren’s syndrome. Adverse Effects: Although ocular toxicity may occur at dosages greater than 250 mg/d for chloroquine and greater than 6.4 mg/kg/d for hydroxychloroquine, it rarely occurs at lower doses. Nevertheless, ophthalmologic monitoring every 12 months is advised. Other toxicities include dyspepsia, nausea, vomiting, abdominal pain, rashes, and nightmares. These drugs appear to be relatively safe in pregnancy. Leflunomide Mechanism of Action: Leflunomide, another csDMARD, undergoes rapid conversion, both in the intestine and in the plasma, to its active metabolite, A77-1726. This metabolite inhibits dihydroorotate dehydrogenase, leading to a decrease in ribonucleotide synthesis and the arrest of stimulated cells in the G1 phase of cell growth. Consequently, leflunomide inhibits T-cell proliferation and reduces production of autoantibodies by B cells. Secondary effects include increases of IL-10 receptor mRNA, decreased IL-8 receptor type A mRNA, and decreased TNF-α–dependent nuclear factor kappa B (NF-κB) activation. Indications and Dosage: Leflunomide is as effective as methotrexate in RA,including inhibition of bony damage. In one study, combined treatment with methotrexate and leflunomide resulted in a 46.2% ACR20 response compared with 19.5% in patients receiving methotrexate alone. Adverse Effects: Diarrhea occurs in approximately 25% of patients given leflunomide, although only about 3–5% of patients discontinue the drug because of this side effect. Elevation in liver enzymes can occur. Both effects can be reduced by decreasing the dose of leflunomide. Other adverse effects associated with leflunomide are mild alopecia, weight gain, and increased blood pressure. Leukopenia and thrombocytopenia occur rarely. This drug is contraindicated in pregnancy. g. Corticotropic Hormones: ACTH. Adrenocorticotropic hormone (corticotropin). Parenteral administration of ACTH 1-39 was reported, more than 50 years ago, to be clinically effective in controlling the symptoms of gouty arthritis. However ACTH 1-39 treatment was rarely used because of the intense suppression of the hypothalamic-pituitary-adrenal (HPA) axis observed following repeated administrations. Mechanism of action. The mechanism of action was initially proposed to explain the anti-inflammatory properties of ACTH1-39. However, recent controlled clinical studies have confirmed the efficacy of ACTH1-39 itself, suggesting the involvement of a mechanism separate from adrenal gland activation. These clinical data highlight the anti-inflammatory properties of ACTH but do not allude to a specific mechanism of action. Systemic administration of ACTH 1-39 produces dose dependent reduction of several parameters of MSU crystal-induced joint inflammation. Dosage: ACTH 1-39 5 μg Adverse effect: Intense suppression of HPA axis 3. Non-pharmacologic Interventions ● ● Healthy Diet ○ Avoid food that may trigger a gout flare, including foods high in purines ( like red meat , organ meat and seafood ), also limit alcohol intake especially the beer and hard liquor instead drink plenty of water. Exercise regularly and lose weight ○ Losing weight reduces pressure on joints. Maintaining a healthy weight can relieve pain, improve function and slow the progression of arthritis. Low impact activities such as walking , bicycling and swimming are easy on the joints, have a low risk of injury and do not put too much stress on the joints. References : ● Gout | Arthritis | CDC) ● Gout - Diagnosis and treatment - Mayo Clinic ● Getting, S.J., Christian, H.C., Flower, R.J. and Perretti, M. 2002. Activation of melanocortin type 3 receptor as a molecular mechanism for adrenocorticotropic hormone efficacy in gouty arthritis. Arthritis & Rheumatism. 46 (10), pp. 2765-2775 ● Katzung’s Basic & Clinical Pharmacology 4th edition