Electrochemistry Worksheet: Redox Reactions & Half-Reactions
advertisement
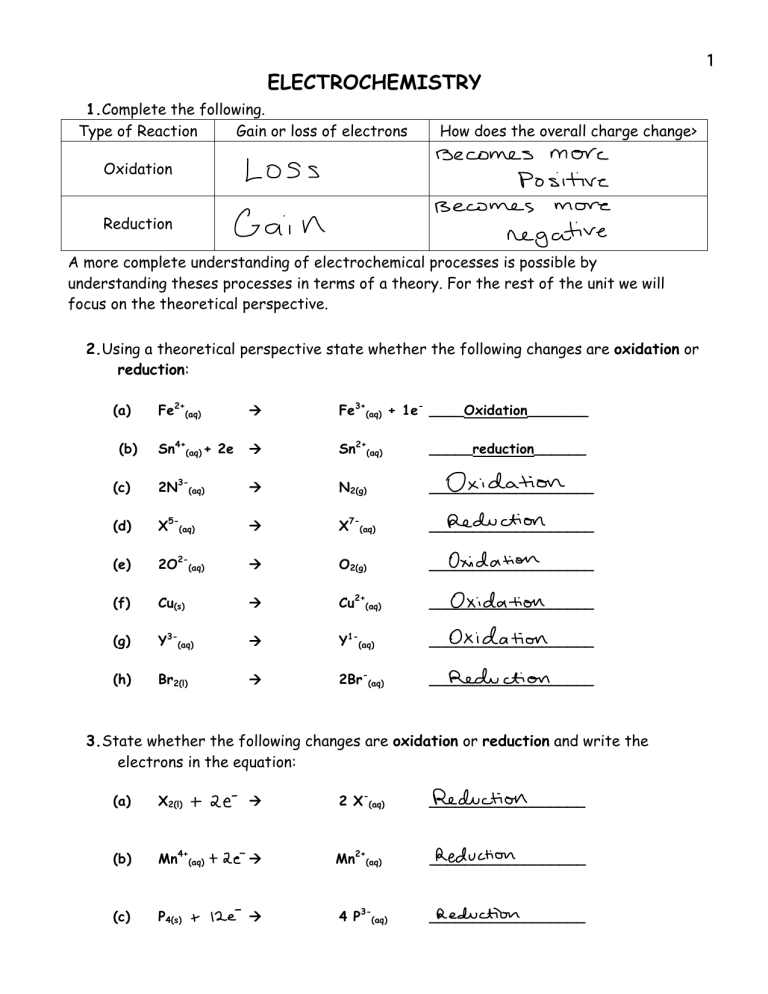
ELECTROCHEMISTRY 1.Complete the following. Type of Reaction Gain or loss of electrons How does the overall charge change> Oxidation Reduction A more complete understanding of electrochemical processes is possible by understanding theses processes in terms of a theory. For the rest of the unit we will focus on the theoretical perspective. 2.Using a theoretical perspective state whether the following changes are oxidation or reduction: Fe2+(aq) ! Fe3+(aq) + 1e- ____Oxidation_______ Sn4+(aq) + 2e ! Sn2+(aq) _____reduction______ (c) 2N3-(aq) ! N2(g) ___________________ (d) X5-(aq) ! X7-(aq) ___________________ (e) 2O2-(aq) ! O2(g) ___________________ (f) Cu(s) ! Cu2+(aq) ___________________ (g) Y3-(aq) ! Y1-(aq) ___________________ (h) Br2(l) ! 2Br-(aq) ___________________ (a) (b) 3.State whether the following changes are oxidation or reduction and write the electrons in the equation: (a) X2(l) ! 2 X-(aq) __________________ (b) Mn4+(aq) ! Mn2+(aq) __________________ (c) P4(s) ! 4 P3-(aq) __________________ 1 2 (d) Z5-(aq) ! Z2-(aq) __________________ (e) 8 S2-(aq) ! S8(s) __________________ (f) Cu+(aq) ! Cu2+(aq) __________________ (g) Al(s) ! Al3+(aq) __________________ (h) 3 S8(s) ! 24 S2-(aq) __________________ (i) Q2-(aq) ! Q2+(aq) __________________ (j) 5 P4(s) ! 20 P-(aq) __________________ (k) J4+(aq) ! J2-(aq) __________________ 4.Write the two half-reactions for each of the following net ionic equations: (a) 2Al(s) (b) Cl2(aq) (c) 3Zn(s) (d) Ba(s) + + + + 6H+(aq) 2Al3+(aq) ! + 2Br-(aq) ! 2Cl-(aq) 2Fe3+(aq) ! 3Zn2+ (aq) 2H+(aq) ! Ba2+(aq) + 3H2(g) + Br2(aq) + H2(g) 2Fe(s) (e) 2Mn7+(aq) (f) 2Al(s) + (g) 2Z4-(aq) + 10Cl-(aq) ! 2Mn2+ (aq) 3Zn2+(aq) ! 2Al3+ (aq) + 3Zn(s) 3Q2+(aq) ! 2Z1-(aq) + Q3(aq) + 5. For each of the following reactions indicate: (a) (b) (c) (d) (a) Br2(aq) (b) Al3+(aq) substance reduced substance oxidized oxidizing agent reducing agent + + (c) 2Ag(s) + (d) 2Al(s) + Sn2+(aq) 3Fe2+(aq) Cu2+(aq) 6H+(aq) 2Br-(aq) ! ! ! ! Sn4+ (aq) + Al(s) + 2Ag+(aq) + 2Al3+(aq) + 3Fe3+(aq) Cu(s) 3H2(g) + 5Cl2(aq) 3 (e) Br2(l) + 2I-(aq) ! I2(aq) (f) Pb2+(aq) + 2K(s) ! 2K+(aq) (g) Ni2+(aq) + Ca(s) ! Ni(s) + + + 2Br-(aq) Pb(s) Ca2+(aq) Introducing the Redox Table 6. Use the table of reduction reactions (Redox Table) from your data booklet to arrange the following metal ions in order of decreasing strength of oxidizing agents: lead (II) ions, silver ions, zinc ions and copper (II) ions. 7. According to the table of redox half reactions, a. What classes of substances usually behave as oxidizing agents? b. What classes of substances usually behave as reducing agents? 8. Earlier in this course, you learned that non-metals to the far right of the periodic table have high electronegativity and were very reactive (like fluorine). You also learned that metals to the far left of the periodic table were the most reactive metals (like potassium or lithium). How does this relate to the position of these substances on the redox table? Relate this to the relative tendency for atoms to attract electrons. 4 5 9. From your knowledge, list two metals that are found as elements and two that are never found as elements in nature. Test your answer by referring to the position of these metals on your redox table. 10. Identify three oxidizing agents form your redox table that can also act as reducing agents. 11. Use your redox table to decide whether the following mixtures would show evidence of a reaction. You do not have to actually write the reaction. a. nickel metal in a solution of silver ions b. zinc metal in a solution of aluminum ions c. an aqueous mixture of copper (II) ions and iodide ions d. chlorine gas bubbled into a bromide ion solution e. an aqueous mixture of copper (II) ions and tin (II) ions f. copper metal in nitric acid 6 Building a Redox Table DEMONSTRATION Procedure: 1. Four sets of the following solutions are prepared. Cu(NO3)2(aq) Zn(NO3)2(aq) Pb(NO3)2(aq) AgNO3(aq) Observations: Cu2+(aq) Cu(s) Zn(s) Mg(s) Ag(s) Zn2+(aq) Mg2+ (aq) Ag+aq) Use the table on the previous page to answer the following questions: 12. Which of the above reactions are spontaneous and which are nonspontaneous? 13. What generalization can be made about a metal and its own aqueous ion? 14. If the forward reaction is spontaneous will the reverse reaction also be spontaneous? 15. List the metallic ions in order of their tendency to form metals, from greatest to least. 16. List the metals in order of their tendency to form positive ions, from greatest to least. 17. How do the lists in questions 15 and 16 compare? 18. Answer the following two questions: a. Write the equations for the reactions that convert the metallic ions to metals. b. These reactions are called HALF-REACTIONS. What kind of halfreactions are these reactions? 7 8 19. Answer the following two questions: a. Write the equations for the reactions that convert the metals to metallic ions. b. What kind of half-reactions are these reactions? 20. Define: a. Oxidation b. Reduction Notice that we can take the two tables of reactivity and combine them into one table: Reactions of ions in order of reactivity: Ag+(aq) Cu2+(aq) Zn2+(aq) Mg2+(aq) + e+ 2e+ 2e+ 2e- Reactions of metals in order of reactivity ! Ag(s) ! Cu(s) ! Zn(s) ! Mg(s) When combined, we get: Ag+(aq) Decreasing Cu2+(aq) order of Zn2+(aq) tendency to gain Mg2+(aq) electrons Zn(s) Mg(s) Cu(s) Ag(s) + e+ 2e+ 2e+ 2e- "! Ag(s) Cu(s) Zn(s) Mg(s) e2e2e2e- <---> <---> <---> <---> "! "! "! ! ! ! ! Zn2+(aq) + 2eMg2+(aq) + 2eCu2+(aq) + 2eAg+(aq) + e- Decreasing order of tendency to lose electrons This is the species most readily reduced or the strongest oxidizing agent Ag+(aq) + Cu2+aq) + Zn2+ (aq) + Mg2+(aq) + Ag(s) Cu(s) Zn(s) Mg(s) This is the species most readily oxidized or the strongest reducing agent 9 Assorted redox questions: 21. Using the data from the following table, rank the oxidizing agents from the strongest to the weakest. W+(aq) X+(aq) Y+(aq) Z+(aq) W(s) NR # # NR X(s) NR NR NR NR Y(s) NR # NR NR Z(s) # # # NR 22. In the redox reaction M + N ! P + Q, which statement is false? (a) If M is the reducing agent, then N is reduced. (b) If M is oxidized, then N is the oxidizing agent (c) If M is oxidized, then N is reduced. (d) If M is the reducing agent, then N is oxidized. 23. A reducing agent is a substance which: (a) loses electrons and becomes reduced (b) gains electrons and becomes reduced (c) loses electrons and becomes oxidized (d) gains electrons and becomes oxidized 24. Build redox tables using the following reaction evidence. a) Co2+(aq) + Zn(s) ! Co(s) + Zn2+(aq) Mg2+(aq) + Zn(s) ! Mg(s) + Zn2+(aq) (no evidence of a reaction, nonspontaneous) b) Be(s) + Cd2+(aq) ! Be2+(aq) + Cd(s) Cd(s) + 2H+(aq) ! Cd2+(aq) + H2(g) Ca2+(aq) + Be(s) ! no evidence of a reaction Cu(s) + 2H+(aq) ! no evidence of a reaction 10 25. The four elements W, X, Y and Z form diatomic molecules and also form ions with a single negative charge. The following observations were made in a series of experiments: 2X-(aq) + Y2(l) ! 2Y-(aq) + X2(l) 2W-(aq) + Y2(l) ! no reaction 2Z-(aq) + X2(l) ! 2X-(aq) + Z2(l) (a) The strongest reducing agent is__________. (b) The strongest oxidizing agent is__________. 26. Metals K, L, M and N and their salts are selectively reacted and yield the following data: L2+(aq) + 2K(s) ! L(s) + 2K+(aq) K(s) + N+(aq) ! no change + K (aq) + M(s) ! K(s) + M+(aq) N(s) + M+(aq) ! N+(aq) + M(s) Using the above data: (a) The strongest oxidizing agent is_________ (b) The strongest reducing agent is _________ (c) The substance most easily oxidized is ________ (d) The substance most easily reduced is _________ (e) The weakest oxidizing agent is___________ (f) The weakest reducing agent is____________ 27. Metals Ga(s), In(s), Mn(s) and Np(s) and their salts are selectively reacted and yield the following data: 3Mn2+(aq) + 2Np(s) ! 3Mn(s) + 2Np3+(aq) In2+(aq) + Ga(s) ! In(s) + Ga3+(aq) Mn2+(aq) + Ga(s) ! no appreciable reaction Using the above data: (a) The strongest oxidizing agent is__________ (b) The substance most readily oxidized is_______ 11 28. Metals M, N, O, and P and their salts are reacted and yield the following results: 2M+(aq) + N(s) ! N2+(aq) + 2M(s) O2+(aq) + N(s) ! no reaction P3+(aq) + 3M(s) ! P(s) + 3M+(aq) (a) The strongest reducing agent is________ (b) The strongest oxidizing agent is________ (c) The weakest reducing agent is _________ (d) The weakest oxidizing agent is_________ (e) The species that attracts electrons most readily is__________ 29. The following reactions were observed to occur: Y2 + X2+ ! 2Y- + X4+ 3Q2- + 2P ! 3Q4- + 2P3+ 2P3+ + 6Y- ! 2P + 3Y2 3X4+ + 3V2- ! V3 + 3X2+ From the above data make a mini redox table. Place the strongest oxidizing agent in the top left hand corner and show the equations undergoing reduction from left to right. Place the electrons in the equations. 30. The elements A, B, C, and D, form diatomic molecules and negative ions. The following observations were made: A2 + 2B- ! D2 + 2C- ! 2A- + B2 no reaction B2 + 2C ! 2B- + C2 (a) The strongest oxidizing agent is_______ (b) The strongest reducing agent is_______ 12 31. The following metals and metallic ions react as follows: A(s) + B+(aq) ! B(s) B(s) + C+(aq) ! no reaction A(s) + C + B(s) + D+(aq) ! C(s) D(s) + + (aq) ! A+(aq) + A+(aq) B+(aq) Using the above data: (a) The strongest reducing agent is ____________ (b) The strongest oxidizing agent is_____________ (c) The substance that undergoes oxidation most readily is______ (d) The substance that undergoes reduction most readily is______ 32. In an oxidation-reduction reaction, electrons are A. gained by the oxidizing agent B. gained by the reducing agent C. lost by the oxidizing agent D. transferred from the oxidizing agent to the reducing agent 33. A reducing agent A. is the product of an oxidation B. becomes reduced C. loses electrons D. gains electrons 34. In a redox reaction, the oxidizing agent A. gains electrons and is oxidized B. loses electrons and is oxidized C. loses electrons and is reduced D. gains electrons and is reduced 35. In the reaction 2Fe3+(aq) + Sn2+(aq) ! Sn4+(aq) + 2Fe2+ aq) ( (a) The oxidizing agent is__________ (b) The reducing agent is__________