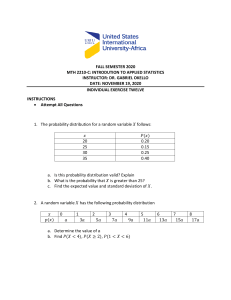
28 June 2020 NVS HYD RO 1 OBJECTIVES After studying this unit, STUDENTS will be able to • Name haloalkanes and haloarenes according to the IUPAC system of nomenclature from their given structures; • Describe the reactions involved in the preparation of haloalkanes and haloarenes and understand various reactions that they undergo; • Correlate the structures of haloalkanes and haloarenes with various types of reactions; • Use stereochemistry as a tool for understanding the reaction mechanism; • Appreciate the applications of organo-metallic compound; • Highlight the environmental effects of polyhalogen compounds. 28 June 2020 NVS HYD RO 2 OUTLINE 1. INTRODUCTION,CLASSIFICATION, NOMENCLATURE 2. PHYSICAL PROPERTIES OF HALO ALKANES & HALO ARENES 3. METHODS OF PREPARATION 4. CHEMICAL PROPERTIES 5. OPTICAL ISOMERISM 6. SN1 & SN2 REACTIONS 7. POLY HALOGEN COMPOUNDS 28 June 2020 NVS HYD RO 3 What is meaning of haloalkane? Organic compounds formed by replacement of one or more hydrogen atom/s by halogen atom/s. from Hydrocarbon Ex: chloromethane, Dichloromethane etc. 28 June 2020 NVS HYD RO 4 Examples for haloalkanes 28 June 2020 NVS HYD RO 5 What is meaning of haloarene? If one or more hydrogen is displaced by halogen atom/s in aromatic ring , product is called as Haloarenes 28 June 2020 NVS HYD RO 6 Importance of Halogen derivative. Haloderivative Organic compounds many importance for Mankind: 1.As solvents for non-polar compounds. 28 June 2020 NVS HYD RO have 2.Chloramphenicol (medicine) to treat typhoid fever. 28 June 2020 NVS HYD RO 8 3.Chloroquine (medicine) used to treat malaria. 4. Some fluorinated compounds are being developed as blood substitute in surgery. 28 June 2020 NVS HYD RO 9 5.Halothane is used as anaesthatic during surgery. 28 June 2020 NVS HYD RO 10 6.THYROXINE is used in the treatment of Goitre disease 28 June 2020 NVS HYD RO 11 7. As starting materials for synthesis of many compounds. 28 June 2020 NVS HYD RO 12 28 June 2020 NVS HYD RO 13 Classification of Haloderivatives Based on Number of Halogen atoms Monohalogen Based on the hybridisation of C−atom of C−X bond of monohalocompounds Compounds containing sp3 C−X bond (X = F, Cl, Br, I). Dihalogen Compounds containing sp2 C−X bond Polyhalogen 28 June 2020 NVS HYD RO Monohalo derivatives 28 June 2020 NVS HYD RO 15 Dihalo derivatives 28 June 2020 NVS HYD RO 16 Dihalo derivatives 28 June 2020 NVS HYD RO 17 Trihalo derivatives 28 June 2020 NVS HYD RO 18 Compounds containing sp3 C−X bond (X = F, Cl, Br, I). 28 June 2020 NVS HYD RO 19 28 June 2020 NVS HYD RO 20 28 June 2020 NVS HYD RO 21 28 June 2020 NVS HYD RO 22 Allylic halides 28 June 2020 NVS HYD RO 23 Benzylic halides 28 June 2020 NVS HYD RO 24 2 sp Compounds containing C−X bond (X = F, Cl, Br, I). 28 June 2020 NVS HYD RO 25 Vinylic halides halogen atom is bonded to a sp2 hybridised carbon atom of a carbon-carbon double bond (C = C). 28 June 2020 NVS HYD RO 2626 Aryl halides halogen atom is directly bonded to the sp2 hybridised carbon atom of an aromatic ring. 28 June 2020 NVS HYD RO 2727 28 June 2020 NVS HYD RO 2828 ORDER OF NAMING Halogen compounds PREFIXES • • • • F Cl Br I Floro Chloro Bromo Iodo 28 June 2020 WORD ROOT • 1C meth • 2C eth • 3C Prop NVS HYD RO PRIMARY SUFIXES • ane • ene • yne 2929 3 1-Bromo + 2 1 prop +ane 1-Bromopropane 28 June 2020 NVS HYD RO 3030 Example-2 28 June 2020 NVS HYD RO 31 Example-3 Name the following: Br Cl 28 June 2020 Cl NVS HYD RO 32 Solution Name the following: Br bromocyclopentane Cl 1,3-dichlorocyclohexane Cl 28 June 2020 NVS HYD RO 33 Q-1 28 June 2020 NVS HYD RO 34 Q-2 28 June 2020 NVS HYD RO 35 Q3 28 June 2020 NVS HYD RO 36 Q-4 28 June 2020 NVS HYD RO 37 Q-5 28 June 2020 NVS HYD RO 38 Q-6 28 June 2020 NVS HYD RO 39 Nature of C-X bond Now THINK why of the following: 1. The C-X bond in haloalkanes is polar in nature. 2. C-X bond length increases as C-F< C-Cl < C-Br <C-I. 3. C-F bond is less reactive as compared to C-I bond. 4. Order of Dipole moment is CH3-Cl > CH3-F> CH3Br >CH3I 5. The dipole moment of chlorobenzene is lower than that of cyclohexylchloride. 28 June 2020 NVS HYD RO 40 28 June 2020 NVS HYD RO 41 PHYSICAL STATE 28 June 2020 NVS HYD RO 42 28 June 2020 NVS HYD RO 43 28 June 2020 NVS HYD RO 44 BOILING POINT 28 June 2020 NVS HYD RO 45 BOILING POINT 28 June 2020 NVS HYD RO 46 BOILING POINT 28 June 2020 NVS HYD RO 47 DENSITY 28 June 2020 NVS HYD RO 48 DENSITY 28 June 2020 NVS HYD RO 49 METHODS OF PREPARATION OF ALKYL HALIDES 1. 2. 3. 4. FROM ALKANE FROM ALKENE FROM ALCOHOL BY HALOGEN EXCHANGE METHOD 28 June 2020 NVS HYD RO 50 1.FROM ALKANE 28 June 2020 NVS HYD RO 51 2.FROM ALKENE 28 June 2020 NVS HYD RO 52 28 June 2020 NVS HYD RO 53 28 June 2020 NVS HYD RO 54 28 June 2020 NVS HYD RO 55 FROM ALCOHOL 28 June 2020 NVS HYD RO 56 3. FROM ALCOHOL 28 June 2020 NVS HYD RO 57 28 June 2020 NVS HYD RO 58 28 June 2020 NVS HYD RO 59 28 June 2020 NVS HYD RO 60 FROM ALCOHOL 28 June 2020 NVS HYD RO 61 28 June 2020 NVS HYD RO 62 4. BY HALIDE EXCHANGE 28 June 2020 NVS HYD RO 63 28 June 2020 NVS HYD RO 64 28 June 2020 NVS HYD RO 65 CHEMICAL PROPERTIES 28 June 2020 NVS HYD RO 66 1.Nucleophilic substitution reactions 28 June 2020 NVS HYD RO 67 Order of reactivity 28 June 2020 NVS HYD RO 68 28 June 2020 NVS HYD RO 69 28 June 2020 NVS HYD RO 70 Williamsons synthesis 28 June 2020 NVS HYD RO 71 28 June 2020 NVS HYD RO 72 28 June 2020 NVS HYD RO 73 28 June 2020 NVS HYD RO 74 28 June 2020 NVS HYD RO 75 Elimination Reactions 28 June 2020 NVS HYD RO 76 28 June 2020 NVS HYD RO 77 28 June 2020 NVS HYD RO 78 Reaction with Mg metal 28 June 2020 NVS HYD RO 79 WURTZ REACTION 28 June 2020 NVS HYD RO 80 Stereochemistry-II Optical Isomerism 28 June 2020 NVS HYD RO 81 Isomers Same molecular formula Constitutional Differ in bond connectivity 28 June 2020 Stereoisomers Differ in three dimensional arrangement NVS HYD RO 82 Stereoisomers Differ in three dimentsional arrangement Configurational Differ in groups or atoms 28 June 2020 NVS HYD RO Conformational Due to free rotation 83 Configurational Differ in groups or atoms Geometrical Due to restricted rotation Optical Due to Chirality Carbon- carbon double bond Carbon- nitrogen double bond Nitrogen-nitrogen double bond Ring Compounds 28 June 2020 NVS HYD RO 84 Optical Isomerism Existence of two or more isomers of a substance that differ in their optical activity Optical activity : the property of a molecule to rotate the plane of plane-polarized light. 28 June 2020 NVS HYD RO 85 28 June 2020 NVS HYD RO 86 28 June 2020 NVS HYD RO 87 28 June 2020 NVS HYD RO 88 What is plane-polarized light ? Ordinary light consists of oscillating electrical and magnetic fields or waves vibrating in all directions perpendicular to the direction the light beam 28 June 2020 NVS HYD RO 89 On passing the ordinary light through a device called Polarizer, its oscillations in all but one direction are blocked and the light emerging from the polarizer oscillates in only one plane P o l a r i z e r This is called plane polarized light 28 June 2020 NVS HYD RO 90 Plane-polarized light Magnetic field Electrical field Plane polarized light Oscillating electrical field The plane polarized light has mutually perpendicular electric and magnetic fields. However, it is the electrical component that is more significant 28 June 2020 NVS HYD RO 91 28 June 2020 NVS HYD RO 92 An optically active substance interacts with the plane polarized light in such a way that the plane of light rotates. P o l a r i z e r 28 June 2020 a Solution of optically active substance NVS HYD RO 93 The substance that rotates the plane of polarized light to the right is called dextrorotatory and the one that rotates it to the left is called laevorotatory Dextrorotatory : rotation to right (The angle of rotation, a, is considered to be positive (+).) Laevorotatory : rotation to left (The angle of rotation, a, is considered to be negative (-).) 28 June 2020 NVS HYD RO 94 28 June 2020 NVS HYD RO 95 LEAVO ROTATORY COMPOUND 28 June 2020 NVS HYD RO 96 ENANTIOMERS 28 June 2020 NVS HYD RO 97 Cause of Optical Activity Optical isomerism is due to chirality in a molecule Greek cheir : hand Chiral objects have a "handedness" The chirality of an object is related to its symmetry rather lack of it 28 June 2020 NVS HYD RO 98 The objects with ‘HANDEDNESS’ cannot be superimposed on their mirror images. e.g., hands or gloves M I R R O R 28 June 2020 NVS HYD RO 99 A right hand cannot be superimposed on a Left hand 28 June 2020 NVS HYD RO 100 Is a scissor chiral ? 28 June 2020 NVS HYD RO 101 The objects that do not have a handedness can be superimposed on their mirror image. e.g., a butterfly M I R R O R The objects that are superimposable on their mirror images are said to be achiral. 28 June 2020 NVS HYD RO 102 A butterfly is symmetric- one half of its body exactly overlaps its other half 28 June 2020 NVS HYD RO 103 Some other examples are: 28 June 2020 NVS HYD RO 104 CHIRAL [ ASYMMETRIC] 28 June 2020 ACHIRAL [SYMMETRIC] NVS HYD RO 105 CHIRAL [ ASYMMETRIC MOLECULES] 28 June 2020 NVS HYD RO 106 Bromochlorofluoromethane M I R R O R 28 June 2020 NVS HYD RO 107 Non-superimposable hence chiral 28 June 2020 NVS HYD RO 108 Compounds that are not superimposable on their mirror images will show optical isomerism These isomers are called enantiomers One of the isomer is dextrorotatory and the other is laevorotatory 28 June 2020 NVS HYD RO 109 In a pair of optically active enantiomers,each enantiomer rotates the plane of polarized light in equal and opposite directions. P o l a r i z e r Solution of Enantiomer 1 Solution of Enantiomer 2 However, a mixture of two in equal proportions will be optically inactive and is called a racemic mixture 28 June 2020 NVS HYD RO 110 28 June 2020 NVS HYD RO 111 Bromochloromethane M I R R O R 28 June 2020 NVS HYD RO 112 The mirror image is superimposable hence the molecule is achiral and does not show optical isomerism 28 June 2020 NVS HYD RO 113 Bromomethane M I R R O R 28 June 2020 NVS HYD RO 114 The mirror image is superimposable hence the molecule is achiral and does not show optical isomerism 28 June 2020 NVS HYD RO 115 Chirality (non-superimposibility of mirror image) is a necessary and sufficient condition for a molecule to show optical activity 28 June 2020 NVS HYD RO 116 Recall the molecule of bromochlorofluoromethane The carbon atom is attached to four different groups. Such a carbon atom is known as asymmetric carbon atom. A molecule possessing asymmetric carbon atom shows enantiomerism. Two optically active isomers (one dextroand other laevorotatory) exist 28 June 2020 NVS HYD RO 117 FISCHER PROJECTION A two dimensional representation of a three dimensional molecule CH3 CH3 CH(CH3)2 H H CH2OH 28 June 2020 CH(CH3)2 CH2OH NVS HYD RO 118 28 June 2020 NVS HYD RO 119 Nucleophilic Substitution A substitution reaction where the reagent is a nucleophile A typical reaction of alkyl halides R X + R Nu Nu + X May follow SN2, SN1 and SNi mechanisms Depends on Nature of substrate Reagent Reaction conditions 28 June 2020 NVS HYD RO 120 SN1 Mechanism SN1 : Substitution Nucleophilic Unimolecular Favoured With neutral or weak Nucleophiles (water, alcohols,amines) (R)3CX 28 June 2020 + (R)3COH H2O NVS HYD RO + HX 121 SN1 Mechanism Unimolecular Rate depends on conc. of ONE species Rate k[RX ] ; Ist Order Depends on alkyl halide and NOT nucleophile Two steps The first step is slow or Rate-determining The second step is the fast step 28 June 2020 NVS HYD RO 122 The SN1 Mechanism Step 1 Cleavage of C-X bond (Formation of carbocation) R3C X -X R3C + ( slow) Step 2 Attack of nucleophile (Formation of product) + R3C + Nu 28 June 2020 R3C NVS HYD RO Nu (fast) 123 The SN1 Mechanism Example (CH3)3CBr + (CH3)3COH H2O + HBr Experimental facts Doubling substrate conc. : Rate doubled Doubling nucleophile conc. : Rate unchanged Thus Rate a [(CH3)3CBr] Rate = k [(CH3)3CBr] 28 June 2020 NVS HYD RO 124 CH3 H3C C CH3 CH3 + Br C H3C + Br CH3 CH3 H2O H3C C CH3 + OH2 + H2O H O+ H3C C OH 3 CH3 28 June 2020 NVS HYD RO CH3 125 Carbocations Reactive intermediates Positively charged carbon Planar (sp2 hybridised) H H C H H C H H H H C > C+ H H C H H > C+ H C H H H H C H C+ H > H H C+ H H H Hyperconjugation stabilises the positive charge 28 June 2020 NVS HYD RO 126 Carbocations are planar Nucleophile can attack from both sides Product is a racemic mixture Rate depends on stability of carbocation Decrease in the order 3o > 2o > 1o Resonance stabilised carbocations favoured (allylic, benzylic) 28 June 2020 NVS HYD RO 127 Order of Reactivity in SN1 Directly related to the stability of intermediate i.e. the Carbonium ion or the Carbocation The order of reactivity is R R R C+ R > H C+ R > H R C+ > H C+ H Tertiary > Secondary > Primary > 28 June 2020 H NVS HYD RO H Methyl 128 SN2 Mechanism SN2 : Substitution Nucleophilic Bimolecular Favoured with strong nucleophiles R R R C X + HO HO R 28 June 2020 R C + Br R NVS HYD RO 129 SN2 Mechanism Bimolecular Rate depends on the conc. of TWO species nucleophile and substrate R HO R C R 28 June 2020 X HO R R R C X R NVS HYD RO HO R C + X R 130 H H H C X + HO HO H H C + Br H Experimental facts Doubling nucleophile conc. : Rate doubles Doubling substrate conc. Thus 28 June 2020 : Rate doubles Rate k[CH 3 Br ][OH ] NVS HYD RO 131 SN2 Mechanism One-step mechanism (Concerted ) Attack of nucleophile and removal of leaving group is simultaneous Leaving group leaves OPPOSITE of the attacking nucleophile 28 June 2020 NVS HYD RO 132 H HO H C H Br HO H H H C Br H HO H C + Br H There is only one transition state 28 June 2020 NVS HYD RO 133 Stereochemistry of SN2 Reaction Attack from side opposite to the leaving group Inversion of Configuration (Walden inversion) 28 June 2020 NVS HYD RO 134 Stereochemistry of SN2 Reaction Attack from side opposite to the leaving group Inversion of Configuration (Walden inversion) 28 June 2020 NVS HYD RO 135 Steric Effect in SN2 Reactions Reactivity 150 28 June 2020 1 0.01 NVS HYD RO 0.0001 136 Reaction with KCN and AgCN CH3CH2 Br + KCN CH3CH2 C + KBr C + N Propanenitrile CH3CH2 Br + CH3CH2 AgCN N KBr Propaneisonitrile 28 June 2020 NVS HYD RO 137 SN1 Vs SN2 Mechanism Unimolecular (two steps) Bimolecular (one step) Rate of Reaction Depends only on the substrate Depends on nucleophile and substrate Nucleophiles Favoured by neutral Nucleophiles 28 June 2020 Favoured by strong Nucleophiles NVS HYD RO 138 SN1 Vs SN2 Solvents Favoured by polar protic solvents Favoured by polar aprotic solvents Alkyl group Favoured by Bulky alkyl groups Favoured by less substituted alkyl groups Stereochemistry Racemic mixture 28 June 2020 Inversion of configuration NVS HYD RO 139 Some Problems 1.Which one of the following will be faster and why? i) CH3Br + HOH CH3OH + HBr CH3OCOCH3 ii) CH3Br + CH3COO- 28 June 2020 NVS HYD RO 140 2. Arrange the following in increasing order of reactivity towards SN1 reactions: i) CH3CH2CH2Br, CH3CH2CH(Br)CH3, (CH3)3CBr ii) 28 June 2020 (CH3)2CHBr , (CH3)2CHCl , (CH3)2CHI NVS HYD RO 141 3. Arrange the following in increasing order of reactivity towards SN2 reactions: i) (CH3)2CHBr , (CH3)3CBr , CH3CH2Br ii) CH3CH2Br , CH3CH2Cl , CH3CH2I 28 June 2020 NVS HYD RO 142 28 June 2020 NVS HYD RO 143 28 June 2020 NVS HYD RO 144 28 June 2020 NVS HYD RO 145 28 June 2020 NVS HYD RO 146 28 June 2020 NVS HYD RO 147 28 June 2020 NVS HYD RO 148 28 June 2020 NVS HYD RO 149 28 June 2020 NVS HYD RO 150 CHEMICAL PROPERTIES OF HALO ARENES 28 June 2020 NVS HYD RO 151 Reasons for the less rectivity of chlorobenzene 28 June 2020 NVS HYD RO 152 Reasons for the less rectivity of chlorobenzene 28 June 2020 NVS HYD RO 153 Reasons for the less rectivity of chlorobenzene 28 June 2020 NVS HYD RO 154 Reasons for the less rectivity of chlorobenzene 28 June 2020 NVS HYD RO 155 O- & p- directing 28 June 2020 NVS HYD RO 156 NUCLOPHILIC SUBSTITUTION REACTION 28 June 2020 NVS HYD RO 157 NUCLOPHILIC SUBSTITUTION REACTION 28 June 2020 NVS HYD RO 158 NUCLOPHILIC SUBSTITUTION REACTION 28 June 2020 NVS HYD RO 159 1.HALOGENATION 28 June 2020 NVS HYD RO 160 2. NITRATION 28 June 2020 NVS HYD RO 161 3.SULPHONATION 28 June 2020 NVS HYD RO 162 4. FRIEDEL-CRAFTS ALKYLATION 28 June 2020 NVS HYD RO 163 REACTION WITH METALS 28 June 2020 NVS HYD RO 164 4. FRIEDEL-CRAFTS ALKYLATION 28 June 2020 NVS HYD RO 165 REACTION WITH METALS 28 June 2020 NVS HYD RO 166 POLY HALOGEN COMPOUNDS 28 June 2020 NVS HYD RO 167 Dichloromethane 28 June 2020 NVS HYD RO 168 Tetrachloromethane 28 June 2020 NVS HYD RO 169 Triiodomethane 28 June 2020 NVS HYD RO 170 Freons 28 June 2020 NVS HYD RO 171 DDT 28 June 2020 NVS HYD RO 172 SUMMARY 1. INTRODUCTION,CLASSIFICATION, NOMENCLATURE 2. PHYSICAL PROPERTIES OF HALO ALKANES & HALO ARENES 3. METHODS OF PREPARATION 4. CHEMICAL PROPERTIES 5. OPTICAL ISOMERISM 6. SN1 & SN2 REACTIONS 7. POLY HALOGEN COMPOUNDS 28 June 2020 NVS HYD RO 173 HOME WORK 1. Why is sulphuric acid is not used during reaction of alcohols with KI? 2. Arrange each set of compounds in order of increasing boiling poings. i) Bromo Ethane , Bromoform, Chloro methane, Dibromo methane (ii) 1-Chloro Propane, Isopropyl chloride, 1-chloro butane 3. Predict the order of reactivity of the following compounds in SN1 & SN2 reactions. (i) The four isomeric bromo butanes ( ii) C6H5CH2Br, C6H5CH2 (C6H5 )Br, C6H5CH2 (CH3)Br, C6H5CH2 (CH3 )(C6H5 )Br 4. Although Chlorine is an electron with drawing group yet it is orho para directing in electrophilic aromatic substitution reactions. Why? 5. Which alkyl halide from the following pairs would you expect to react more rapidly by SN2 mechanism? Explain your answer? (A) CH3-CH2-CH2-CH2-Br & CH3-CH2-CHBr –CH3 28 June 2020 HYD RO 174 (B)CH3-CH2-CHBr –CH3 NVS & C(CH 3)3Br HOME WORK 6. alo alkanes react with KCN to form alkyl cyanides as main product while AgCN forms isocyanides as the chief product. Explain. 7. COMPLETE THE FOLLOWING 28 June 2020 NVS HYD RO 175 HOME WORK 8. Explain the following named reactions (a) Williamson’s synthesis (b) Swarts reaction ©Finkelstein reaction (d)Sand mayers reaction (e) Gatterman reaction (f)Fittig reaction 9.What are ambident nucleophiles? Explain with an example. 10.What happens when (i) n-butyl chloride is treated with alcoholic KOH (ii) Bromobenzene is treated with Mg in the presence of dry ether (iii) Chlorobenzene is subjected to hydrolsis. (iv) Ethyl chloride is treated with aqueous KOH (v)28 June Methyl KCN? 2020 chloride is treated with NVS HYD RO 176 28 June 2020 NVS HYD RO 177