States of Matter: Gases, Liquids, Solids & Intermolecular Forces
advertisement

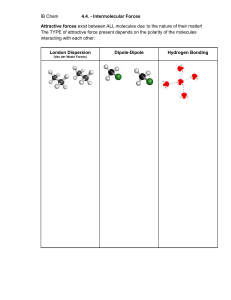
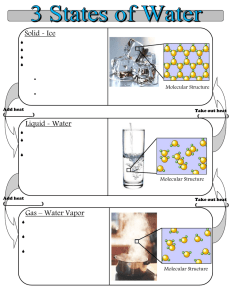
I – The three states of matter. A single body may exist in three different physical states: gaseous, liquid and solid. The existence of these three states is due to the balance between intermolecular forces and the molecular agitation which tends to separate them from each other and to allocate them randomly. If the average energy of agitation is much higher, same order of magnitude, or smaller than the average energy of interaction between molecules, the body is a gas, liquid or solid respectively. 1- Molecular Agitation The idea of molecular agitation was suggested by the existence of Brownian motion. If we look to an aqueous suspension of fine particles, pollen grains for example, through the microscope we see that each particle moves tirelessly in all directions. The movement is essentially irregular. The speed and range of motion depend on the particle size (they are inversely proportional to it). This permanent agitation of the molecules, called thermal agitation is most complex in the case of fluids (gas and liquid) because it combines translation, rotation of the molecule around its center of gravity and vibration of atoms within the molecule itself. In a gas, the distance traveled by a molecule as a result of thermal agitation, before colliding with another, is very large compared to the molecular volume. In a liquid, it is of same order of molecular dimensions. In a solid, the molecules can just oscillate with small amplitude. 2- Binding forces between molecules. Some properties of liquids, solids and gases depend on the existence of molecular interaction forces. For example, increasing the magnitude of these forces in a real gas explains why it doesn’t strictly follow the laws of ideal gas under high pressure. These forces do not lead to the formation of chemically stable compounds. They only intervene on certain physical properties such as boiling, viscosity or surface tension. Finally, they all are of electrostatic nature. According to the value of the binding energy (potential energy of interaction), we differentiate: - The forces of Van der Waals (< 8kJ/mol) - The hydrogen bond (8 to 50 kJ/mol) a- The forces of Van der Waals : They consist of three aspects and their effects are cumulative: - The interaction of Keesom which occurs between two neighboring polar molecules. Due to their permanent dipole moment, when these two molecules are close and in a positive direction, they attract each other by their ends of opposite signs. Keesom proved that the binding potential energy has the following form: 2 1 4 Uk 2 3 4 0 r 6 kT Where r is the average distance (in meters) between two molecules, µ their dipole moment (C.m), T the temperature in K and k the Boltzmann constant (k = 1.3805 1023 J.K-1). From the formula any increase in temperature, promoting molecular agitation decreases this kind of interaction. - The interaction of Debye which occurs between a polar molecule and a nonpolar one. The permanent dipole moment of the polar molecule, influencing the electronic distribution of nonpolar molecule, induces its polarization. The two dipoles can interact as previously. Debye proved that the binding potential energy has the following form: 1 2 2 UD 4 0 r 6 Where α is the polarizability of the nonpolar molecule, µ its dipole moment. - The interaction of London which occurs between two nonpolar molecules. The intermolecular binding energy is greater than the sum of the two previous ones. Thus, there exists a third type of interaction. At certain time, as a result of the continual movement of electrons around the nucleus, the centers of masses of positive and negative charges may no longer coincide, giving a polar structure to the molecule. This instantaneous dipole is then able to induce a second dipole on neighboring molecule and in the favorable cases to interact with it. The binding potential energy is of the following form : 3 1 I 2 UL 4 4 0 r 6 Where I is the instantaneous dipole moment. b- Hydrogen bonds It is also an electrostatic interaction of dipole-dipole between: - On one hand, hydrogen linked by a strong bond (covalent) to another strongly electronegative atom. (F, O, N…) - On the other hand, another electronegative atom. The energy is slightly higher than a bond of Van der Waals. Due to its high electronegativity, the oxygen atom attracts partially the electrons of the bond. A partial positive charge appears on the hydrogen atom that can attract a lone pair of another neighbor oxygen atom. II- The gaseous state. The gases are fluids which have no form or specific volume. Their molecules are in continuous fast motion. Noting that their direction varies as a result of intermolecular collisions and collisions with the container's walls which limit them. These shocks are the source of the pressure exerted by a gas on the walls. Summary of macroscopic laws of gases -Mariotte law : PV = RT. It only applies properly to gases approaching the ideal gas (gas of low molecular weight, low pressure and high temperature) if not, the real gases obey the law of Van der Waals. a P 2 V b RT V a P 2 V Internal pressure of a real gas or cohesion pressure b is the covolume : The free volume v available for molecular motion is less than the volume V occupied by gas. We call covolume the difference b between V and v : v = V - b. -Law of Gay-Lussac : At constant volume PT-1 = cte T P P0 1 273 . 15 -Law of Charles : At constant pressure VT-1 = cte T V V0 1 273 . 15 -Law of Avogadro: At constant temperature and pressure, one mole of gas occupies the same volume regardless of the gas. At normal temperature and pressure (0°C et 1 Atm) this volume is 22.4 L. -Law of Dalton: The pressure of a mixture of gases is equal to the sum of partial pressures that the gases would exert if each separately occupied all the available volume. RT Pi ni V P Pi i Mole fraction : i ni ni Pi i P -Law of Henry (gas solubility): At equilibrium, the concentration C of a gas dissolved in a liquid is proportional to the partial pressure « p » of the gas above the liquid: C=kp The constant k depends on the gas/liquid couple and varies with temperature. III- The solid state The solid state is a condensed state characterized by its rigidity. 1- The organization of solid. ab- Crystals (See Inorganic Chemistry course) Glasses (See Inorganic Chemistry course) c- Polymers These are natural organic compounds (cellulose, starch rubbers, proteins such as Keratin or collagen) or artificial (polyamides (nylon), polyesters (tergal), polyvinyl, silicones). Crystalline structure can be observed, but more often they are organized in long chains more or less parallel and associated with each other. The mechanical properties of these solids (particularly their plasticity) vary greatly with molecular structure. d- The composite solid Many solids found in nature or manufactured combine crystals, glasses and polymers. This is for example the case of cartilage composed of a glassy substance (mucopolysaccharides of fundamental substance) reinforced by polymer fibers (collagen). The cartilage is therefore flexible and malleable. During the growth, crystals of calcium phosphate impregnate the fundamental substance. The cartilage becomes a bone stronger and stiffer. 2- Cohesive forces. Whatever the structure of the solid is, their cohesion is ensured by the interatomic or intermolecular forces according to their nature, we differentiate: Atomic or covalent crystals (diamonds). All atoms are joined together by strong covalent bonds. Ionic crystals; electrostatic forces. Metallic crystals; atoms are linked together with all the delocalized electrons in the metal. Molecular crystals; force of Van Der Waals and hydrogen bonds. Cohesion of glasses and polymers is provided by covalent and Van Der Waals bonds. 3- Electrical properties of crystals. See Inorganic chemistry course (Insulator, conductor, semi-conductor….) IV- Liquid state The liquid state is considered to be the intermediate between gaseous and solid states. It has properties in common with each of them: it’s a fluid like gases (liquid also obeys the same equation of Van der Waals than its vapor); it’s a condensed state like solids (intermolecular distances are very small). V- The liquid crystalline state We could manufacture ultrathin and flexible screens for computers and televisions with a kind of material which seems to be neither solid nor liquid. Liquid crystals are substances that flow like viscous liquids, but whose molecules are arranged almost like those of a crystal. These substances are an intermediate state of the matter (mesophase state) with the fluidity of a liquid and the molecular order of a solid. The typical molecules of liquid crystals are long molecules in the form of bars, an example is p-azoxyanisol : H3C O N O N O CH3 The bar shape allows the molecules to stack over each other like raw spaghetti. They are parallel, and they can slide over each other in the direction of their long axis. Because of this order, the liquid crystals are anisotropic. The properties of anisotropic material depend on the direction in which the measurement is made. The viscosity of liquid crystals is minimal in the parallel direction to that of molecules. It takes less energy to their long molecules in the shape of bars to slide over each other in the direction of axes, rather than rolling over each other in the perpendicular direction. Isotropic material properties do not depend on the direction of measurement. Ordinary liquids, for example, are isotropic: the viscosity is the same in all directions. Liquid crystals become isotropic liquids when heated beyond a certain temperature because their molecules will have enough energy to overcome the attractive forces which restrict their movements. There are three kinds of liquid crystals which differ in the arrangement of their molecules: In a nematic phase (Greek word meaning thread), molecules are placed next to each other, but they are shifted like cars on a multi-line highway. In a smectic phase (Greek word meaning soapy, lamellar), the molecules are lined up like soldiers on parade, and form layers. In a cholesteric phase (cholesterol is a Greek term meaning solid bile), the molecules form nematic layers, but molecules of two adjacent layers are oriented in different directions, so that the arrangement of molecules in the liquid crystal is helical. a)smectic liquid crystal, b) nematic liquid crystal, c) Helical nematic liquid crystal (cholesteric) We can also classify the liquid crystals according to their mode of preparation: Thermotropic liquid crystals are obtained by melting a solid phase. The liquid crystal phase exists in a temperature range between the solid and liquid phase. p-azoxyanisol is a thermotropic liquid crystal. Thermotropic liquid crystals are very viscous and can be translucent or opaque. They are used in watches, computer screens and thermometers. Lyotropic liquid crystals result from the action of a solvent on a solid such as: cleaning solutions, lipids (fat) and cell membranes. These molecules like lauryl sulfate (C12H25OSO3- Na+ a detergent) are composed of nonpolar long chains of hydrocarbons having a polar end. OSO3- Na+ Dilute solutions of detergents (or surfactant) tend to form layer on the surface of water. The detergent molecules are aligned next to each other at the surface of water with their polar heads in water and their nonpolar tails in the air. At high concentrations, the detergents form micelles, small clusters where the polar heads of the micelles link to water outside of the micelle, and the hydrocarbon tails towards the interior of the micelle. The hydrocarbon tails of the detergent molecules are attracted by fat impurities and surround them by a micellar structure. The polar heads, at the outer surface of the micelle, allow it to remain suspended in the water and consequently gather fats. Lipids which form cell membranes are structurally similar to that of micelles. When mixed with water, they spontaneously form very thin layers where the molecules are aligned in rows and form a double layer. The polar heads are directed outwards, on both sides of the double layer. These sheets (double layer) form the protective membranes of our cells. Electronic displays use the fact that the orientation of liquid crystal molecules changes in the presence of an electric field. This reorientation causes a variation in their optical properties which make them opaque or transparent, creating as well a drawing on the screen. The interest of cholesteric liquid crystals is that their helical structure unfolds when the temperature varies slightly. As the torsion of their helical structure affects their optical properties, these will vary with temperature; this fact is used in liquid crystal's thermometers.