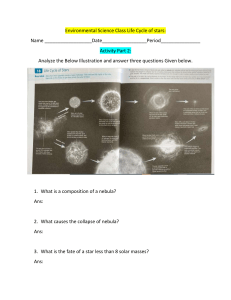
Why ionic compounds have high melting and boiling point? Explain in terms of bonding and structure. Sample Question: Explain, in terms of its structure and bonding, why magnesium oxide has a very high melting point. (4 marks) Ans: Magnesium Oxide is giant ionic structure, it contains Mg2+ and O2- ions, there is high attraction between the oppositely charged ions, takes lots of energy to break the ionic bonds. Why Diamond/Graphite/SiO2 (Silicon dioxide) does has high melting and boiling points (3 marks)? Ans: Diamond/Graphite/SiO2 is giant covalent structure, there are strong covalent bonds throughout the whole structure, which takes lots of energy to break. Write down two features of dynamic equilibrium. (2 marks) Ans: i) The rate of forward and backward reaction are equal ii) The concentration of reactant and products are constant. Explain in terms of bonding why heat is released in exothermic reaction? (3 marks) Ans: Energy is released when bonds are made, energy is absorbed when bonds are broken, in an exothermic reaction more energy is released during bond making than absorbed while bond breaking. (Practice: Explain in terms of bonding why heat is absorbed in endothermic reaction?) Explain why cracking is important in the oil industry? (4 marks) Ans: Cracking produces smaller hydrocarbons, smaller hydrocarbons have greater demand, they are easier to burn, and also cracking produces more reactive alkenes. Why SiO2 does reacts with CaO? What type of reaction is this? (2 marks) Ans: They react because SiO2 is acidic while CaO is basic. This is a neutralization reaction. Explain in terms of electron transfer, what happens when Magnesium and Chlorine reacts (ionic bonding) Ans: Magnesium loses 2 electrons to form a Mg2+ ion, 2 chlorine atoms gains one electron each to form two Cl– ions. There is attraction between the oppositely charged ions. Practice: Na reacting with Cl, Na reacting with O. Why does elements in the same group has similar chemical properties (e.g: Na and K, Cl and Br) Ans: Because they have the same number of electrons in the outer shell. What is meant by R.A.M (Relative Atomic Mass) Ar? (2 marks) Ans: It is the average mass of all the isotopes of an element. The mass of each isotope is in the scale of Carbon-12. How to do a flame test on solid M (example)? (3 marks) Ans: Take a clean nichrome wire, take solid M on the tip of the wire, then using the wire put the solid M in the blue part of the Bunsen burner flame. Why does solid Lead (II) Bromide does not conduct electricity? (1 mark) Ans: In solid state the ions are held within a lattice and are not free to move and conduct electricity. State two characteristics of the compounds in a homologous series. (2 marks) Ans: Choose any two from these: (a) having the same functional group (b) having the same general formula (c) differing from one member to the next by a –CH2– unit (d) displaying a trend in physical properties (e) sharing similar chemical properties What is meant by isomers? (2 marks) Ans: It means compounds having same molecular formula but different structural formula. What is the compound present in rust? (1 mark) Ans: Hydrated Iron (III) Oxide Name two substances that iron reacts with when it rusts? (2 marks) Ans: Air/Oxygen and Water. State two methods of preventing rusting? (2 marks) Ans: Choose any two Galvanizing, Painting, alloying, coating with tin. What does the symbol ⇌ indicate about the reaction? (1 mark) Ans: The reaction is reversible. Describe a chemical test to show that a liquid is water/ contains water. ( 2 marks) Ans: Add few drops of the liquid to anhydrous copper(II)sulfate, if the liquid is water/contains water, it will turn from white to blue. Add a few drops of liquid to anhydrous cobalt(ii)chloride. if the liquid is water/contains water, it will turn from blue to pink. Describe a physical test to show that the water is pure. (2 marks) Ans: Take sample of the water and heat it, if it boils at 100°C then water is pure. What is meant by hydrocarbon? (2 marks) Ans: Compounds made of hydrogen and carbon only. How a covalent bond holds two atoms together? (2 marks) Sample Question 1: Explain how the covalent bonds in the water molecule hold the hydrogen and oxygen atom together? (2 marks) Ans: The positive nuclei of both the oxygen and hydrogen atom are attracted towards the shared electron pair. Sample Question 2: Explain how hydrogen and bromine atoms are held together in a molecule of hydrogen bromide? (2 marks) Ans: The positive nuclei of both the hydrogen and bromine atom is attracted towards the shared electron pair. Explaining why a simple molecular substance has lower m.p/b.p than an ionic substance. (2 marks) Sample Question: Explain why water has a much lower melting point than sodium oxide. (2marks) Ans: There are weak intermolecular attraction between the water molecules, which takes low energy to overcome. There is high attraction between the Na + and O2- ions, which take lots of energy to break the ionic bonds. Raw materials for Haber Process Sample Question: State a raw material used as the source of each gas. (2 marks) Nitrogen: Air Hydrogen: Natural Gas What catalyst is used in haber process? Ans: Iron Why is a metal malleable? (2 marks) Sample Question: Why is iron malleable? (2 marks) Ans: Iron is malleable because when force is applied the particle/cations/ions (do not write molecules!!) are able to slide over each other. Why is a metal good conductor of electricity? (2 marks) Sample Question: Why is iron a good conductor of electricity? (2 marks) Ans: Iron structure has delocalized electrons, which are able to move around and conduct electricity. What are the colors of fluorine, chlorine, bromine and iodine at room temperature? (4 marks) Ans: Fluorine: Yellow Chlorine: Green Bromine: Reddish brown Iodine: black What is the state of fluorine, chlorine, bromine and iodine at room temperature? ( 4 marks) Ans: Fluorine: gas Chlorine: gas Bromine: liquid Iodine: solid What is meant by the terms unsaturated? ( 1 mark) Ans: Contains carbon to carbon double bond. What are the raw materials for blast furnace? (3 marks) Ans: Iron ore/Haematite/Fe2O3 Coke/carbon/C Limestone/CaCO3 What are the main ores of iron and aluminium? (2 marks) Ans: Aluminium: Bauxite (Al2O3) What is meant by the term catalyst? (2 marks) Ans: a catalyst increases the rate of a reaction by decreasing the activation energy, Ea, of a reaction, and is unchanged at the end of a reaction State two similarities and two differences between the reactions of lithium (or Sodium) and Potassium with water. (4 marks) Ans: Similarities: 1. Both metals darts on the surface of the water. 2. In both reactions bubbling of invisible gas takes place. Differences: 1. Potassium burns with a lilac flame, whereas no flame is seen for lithium/Sodium 2. Potassium reacts more vigorously than lithium/Sodium. 3. What is meant by the term exothermic? (1 mark) Ans: A reaction in which heat is released. Explain how a catalyst works? (2 marks) Ans: A catalyst provides an alternative route for the reaction to occur which has less activation energy. Explain, in terms of particles, why an increase in temperature increases the rate of reaction. (3 marks) Ans: At higher temperature, particles move faster collision frequency increases. A greater number of particles have higher energy than the activation energy, hence greater number of successful collisions occurs per second . Which hydrocarbon is the main component of natural gas? (1 mark) Ans: Methane (CH4) What is meant by the term cracking? (2 marks) Ans: It is a process in which large hydrocarbons are broken down to smaller hydrocarbons with the help of catalyst or heat. Give a test to show that a hydrocarbon is unsaturated? (2marks) Ans: Unsaturated hydrocarbons turns bromine water from orange to colourless. State two changes that occur in the formation of addition polymerization from its monomer. Ans: 1. The carbon to carbon double bond breaks 2. The monomers join to form a big molecule Describe how crude oil is separated into fractions in industry. (4 marks) Ans: By using a fractionating column, the crude oil is heated and passed into tower. Fractions condense at different levels of the towers, as different fractions have different boiling points. What are the general formula for alkanes and alkenes? ( 2 marks) Ans: Alkane: CnH2n+2 Alkene: CnH2n What is meant by the term biodegrade? (2 marks) Ans: Breaks down/decomposes/decays by bacteria/microbes/microrganisms. Description of making an insoluble salt. (5 marks) Sample Question: Describe how she could use solutions of lead (II)nitrate and sodium bromide to obtain a pure, dry sample of lead(II)Bromide. (5 marks) Ans: Mix the solutions of lead (II) nitrate and sodium bromide in a beaker, solid lead (II) bromide is formed. Filter to remove solid lead (II) bromide. Wash it with distilled water. Then leave it to dry. What are isotopes? (2 marks) Ans: Isotopes are atoms of the same element with the same proton number but different neutron number. Describe two observations made when magnesium burns in air (2 marks) Ans: i) Magnesium burns with a bright white flame. ii) A white solid is formed Metal Oxides are basic while non-metal oxides are acidic. Eg. MgO – basic oxide Na2O- basic oxide SO2 – acidic oxide CO2– acidic oxide Incomplete combustion of hydrocarbons and its products. Sample Questions: State the condition that causes incomplete combustion. (1 mark) Ans: lack of supply of air/oxygen. Identify the poisonous gas produced. (1 mark) Ans: Carbon monoxide Explain why this gas is poisonous. (2 marks) Ans: It attaches more efficiently with haemoglobin than oxygen, hence hinders the transportation of oxygen. What problem is caused by releasing NO2/SO2 in the atmosphere? (2 marks) Ans: It causes acid rain, acid rain causes plants to die/damages monuments made of limestone/ kills fish/ increases soil pH. Reason the polymer does not biodegrade. Sample question: What property of these polymers prevents them from biodegrading? ( 1 mark) Ans: They are inert/unreactive. Why use phenolphthalein/ methyl orange indicator in titration rather than a universal indicator? Ans: Methyl orange/phenolphthalein indicators gives a sharp colour change whereas universal indicator does not. Describing the structure and bonding in a metal. Sample question : Indium is a metal in group 3 of the periodic table . Describe the structure and bonding in indium (3 marks) Ans: Indium structure has cations (positive metal ions) and delocalized electrons, there is high attractions between the cations and delocalized electrons. Show that a substance is a catalyst, not a reactant. ( 2 marks) Remember: Catalyst increases the rate of reaction, without getting used up itself, so their mass will be unchanged. Sample question: In the first experiment the student added 1 g of solid A. Describe what he could do with the contents of the conical flask at the end of the experiment to show that A was a catalyst, and not a reactant. (2 marks) Ans: Separate solid A and weigh, if the mass is the same as before then it’s a catalyst. Given that at least one of the reactant is gaseous, What happens when the pressure is increased. Made up question: A(g) + B(g) → C(g) + D(g) What happens to the rate of reaction when pressure is increased? ( 1 mark) Ans: Rate of reaction increases. Explain your prediction in terms of the particle collision theory. (2 marks) Ans: At higher pressure particles are closer together, collision frequency increases, hence rate of reaction increases. Why is graphite soft and slippery? Sample question: Explain, in terms of its structure, why graphite can act as lubricant (2 marks) Ans: In graphite when force is applied, the layers can slide over each other. Why graphite conducts electricity? Sample question: The structure of graphite has one feature in common with that of the metals. This feature allows graphite to conduct electricity. Suggest what is this feature is and why it allows graphite to conduct electricity. Ans: Graphite structure has delocalized electrons, which can move around to conduct electricity. Pb(NO3)2 (aq) + 2NaN3(aq) → Pb(N3)2 (s) + 2NaNO3(aq) What is the name given to this type of reaction? (1 mark) Ans: precipitation reaction Observations when acid is added to a metal carbonate. Sample question: State two observations you would make when dilute nitric acid is added to solid lead (II) carbonate Ans: 1. Bubbling of invisible gas 2. Solid lead(II) carbonate shrinks Test for chlorine Sample question: Describe a chemical test to show that the gas evolved at the positive electrode is chlorine. (2 marks) Ans: Chlorine gas bleaches a damp litmus paper. Suggest a suitable element for inert electrodes. ( 1 mark) Ans: carbon/platinum Give a test for carbon dioxide? (2 marks) Ans: carbon dioxide turns limewater milky. Name the process in which glucose is converted to ethanol. ( 1 mark) Ans: Fermentation What is the purpose of the yeast in fermentation? Ans: it acts as the catalyst.