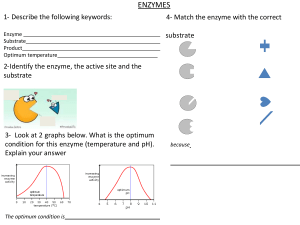
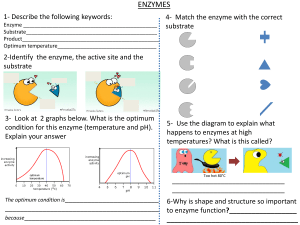
o o BIOCHEMISTRY: ENZYMES AND VITAMINS GENERAL CHARACTERISTICS OF ENZYMES ENZYMES are usually protein that act as biological catalysts Each cell in the human body contains thousands of different enzymes Enzymes cause cellular reactions to occur millions of times faster that corresponding uncatalyzed reactions An enzyme speeds a reaction by lowering the activation energy, changing the reaction pathway that provides a lower energy rout for conversion of substrate to product As catalysts enzymes are not consumed in the reactions A few enzymes are now known to be ribonucleic acids (RNA) ENZYME STRUCTURE Most enzymes are globular proteins Two general structural classes: SIMPLE ENZYME CONJUGATED ENZYME Composed only of protein (amino acid chains) o It is the 3˚ structure of the simple enzymes that makes it biologically active Has a non-protein part in addition to a protein part 1. Apoenzyme – the protein part of a conjugated enzyme 2. Cofactor – the nonprotein part of a conjugated enzyme (bound to the enzyme for it is to maintain the correct configuration at the active site The substrate is the substance upon which the enzyme “acts” E.g. in the fermentation process sugar is converted to alcohol, therefore in this reaction sugar is the substrate THREE IMPORTANT ASPECTS OF THE NAMING PROCESS 1. Suffix –ase identifies it as an enzyme → E.g. urease, sucrose, and lipase are all enzyme designations → Exception: the suffix –in is still found in the names of some digestive enzymes, e.g., trypsin, chymotrypsin, and pepsin 2. Type of reaction catalyzed by an enzyme is often used as a prefix → E.g. oxidase – catalyzes an oxidation reaction → E.g. hydrolase – catalyzes a hydrolysis reaction 3. Identity of substrate is often used in addition to the type of reaction → E.g. glucose oxidase, pyruvate carboxylase, and succinate dehydrogenase SIX MAJOR CLASSES o Enzymes are grouped into six major classes based on the types of reaction they catalyze CLASSES OXIDOREDUCTASES TRANSFERASES 3. 4. Holoenzyme – the biochemically active conjugated enzyme produced from an apoenzyme and a cofactor Coenzyme – a small organic molecule that serves as a cofactor in a conjugated enzyme NOMENCLATURE AND CLASSIFICATION OF ENZYMES Most commonly named with reference to their function o Type of reaction catalyzed o Identity of the substrate A substrate is the reactant in an enzyme-catalyzed reaction: HYDROLASES SELECTED SUBCLASSES & TYPE OF REATION CATALYZED Oxidases → Oxidation of a substrate Reductases → Reduction of a substrate Dehydrogenases → Introduction of double bond (oxidation) by formal removal of two H atoms from substrate, the H being accepted by a coenzyme Transminases → Transfer of an amino acid group between substrate Kinases → Transfer of a phosphate group between substrates REACTION CATALYZED an enzyme that catalyzes an oxidation– reduction reaction Lipases → Hydrolysis of ester linkages in lipids Proteases → Hydrolysis of amide linkages in proteins Nucleases → Hydrolysis of sugarphosphate ester bonds in nucleic acids Carbohydrases → Hydrolysis of glycosidic bonds in carbohydrates an enzyme that catalyzes a hydrolysis reaction in which the addition of a water molecule to a bond causes the bond to break an enzyme that catalyzes the transfer of a functional group from one molecule to another LYASES Phosphatases → Hydrolysis of phosphateester bonds Dehydratases → Removal of H2O from a substrate Hydratases → Addition of H2O to a substrate Decarboxylases → Removal of CO2 from a substrate Deaminases → Removal of NH3 from a substrate ISOMERASE Racemases → Conversion of D isomer to L isomer or vice versa Mutases → Transfer of a functional group from one position to another in the same molecule LIGASES Synthetases → Formation of new bond between two substrates, with participation of ATP Carboxylases → Formation of new bond between a substrate and CO2, with a participation of ATP an enzyme that catalyzes the addition of a group to a double bond or the removal of a group to form a double bond in a manner that does not involve hydrolysis or oxidation ENZYME-SUBSTRATE COMPLEX: the intermediate reaction species that is formed when a substrate binds to the active site of an enzyme o Intermediate reaction species formed when substrate binds with the active site o Needed for the activity of enzyme o Orientation and proximity is favorable and reaction is fast TWO MODELS FOR SUBSTRATE BINDING TO ENZYME o Lock-and-Key Model: Active site in the enzyme has a fixed, rigid geometrical conformation Only substrate of specific shape can bind with the active site; a substrate whose shape and chemical nature are complementary to those of the active site can interact with the enzyme Fails to take into account protein’s conformational changes to accommodate a substrate molecule There are weak binding forces (R group interactions) between parts an enzyme that catalyzes the isomerization (rearrangement of atoms) of a substrate in a reaction, converting it into a molecule isomeric with itself an enzyme that catalyzes the bonding together of two molecules into one with the participation of ATP o Induced Fit Model: Substrate contact with enzyme will change the shape of the active site Allows small change in space to accommodate substrate The enzyme active site, although not exactly complementary in shape to that of the substrate, is flexible enough that it can adapt to the shape of the substrate MODELS OF ENZYME ACTION Explanations of how enzymes function as catalysts in biochemical systems are based on the concepts of an enzyme active site and enzyme-substrate complex ACTIVE SITE: relatively small part of the enzyme’s structure that is actually involved in catalysis o Where substrate binds to enzyme o Formed due to folding and bending of the enzyme o Usually a “crevice like” location in the enzyme o Some enzymes have more than one active site FORCES THAT DETERMINE SUBSTRATE BINDING o H-bonding o Hydrophobic interaction o Electrostatic interactions TEMPERATURE Higher temperature results in higher kinetic energy which causes an increase in number reactant collisions, therefore there is higher activity Optimum temperature: temperature at which the rate of enzyme-catalyzed reaction is maximum Optimum temperature for human enzymes is 37˚C Increased temperature (high fever) leads to decreased enzyme activity In high-temperature, high-pressure vessels called autoclaves, super-heated steam is used to produce a temperature sufficient to denature bacterial enzymes ENZYME SPECIFICITY ENZYME SPECIFICITY: the extent to which an enzyme’s activity is restricted to a specific substrate, a specific group of substrates, a specific type of chemical bond, or a specific type of chemical reaction TYPES OF ENZYME SPECIFICITY o Absolute Specificity An enzyme will catalyze a particular reaction for only one substrate This is most restrictive of all specificities (not common) E.g. Catalase is an enzyme with absolute specificity for hydrogen peroxide (H2O2); Urease absolute specificity for urea o Stereochemical Specificity An enzyme can distinguish between stereoisomers Chirality is inherent in an active site (amino acids are chiral compounds) L-amino acid oxidase – catalyzes reactions of L-amino acids but not D-amino acids o Group Specificity Involves structurally similar compounds that have the same functional groups E.g. Carboxypeptidase: cleaves amino acids one at a time from the carboxyl end of the peptide chain o Linkage Specificity Involves a particular type of bond irrespective of the structural features in the vicinity of the bond Considered most general of enzyme specificities E.g. Phosphatases: hydrolyze phosphate – ester bonds in all types of phosphate esters FACTORS THAT AFFECT ENZYME ACTIVITY ENZYME ACTIVITY - a measure of the rate at which an enzyme converts substrate to products in a biochemical reaction FOUR FACTORS AFFECT ENZYME ACTIVITY pH Substrate concentration Drastic changes in pH can result in denaturation of proteins Optimum pH: pH at which enzyme has maximum activity Most enzymes have optimal activity in the pH range of 7.0-7.5 Exception: digestive enzymes o Pepsin: optimum pH =2.0 o Trypsin: optimum pH = 8.0 At constant enzyme concentration, the enzyme activity increases with increased substrate concentration Enzyme saturation: the concentration at which it reaches its maximum rate and all of the active sites are full Turnover number: number of substrate molecules converted to product per second per enzyme molecule under conditions of optimum temperature and pH Enzyme concentration Enzymes are not consumed in the reactions they catalyze At a constant substrate concentration, enzyme activity increases with increase in enzyme concentration o The greater the enzyme concentration, the greater the reaction rate o Noncompetitive inhibitors: do not compete with the substrate for the same active site Binds to the enzyme at a location other than active site REVERSIBLE COMPETITIVE INHIBITION REVERSIBLE NONCOMPETITIVE INHIBITION EXTREMOZYMES EXTREMOZYME – o a microorganism that thrives in extreme environments, environments in which humans and most other forms of life could not survive o high interest for industrial chemists enzymes are heavily used in industrial processes industrial processes require extremes of temp., pressure, and pH EXTREMOPHILE - a microorganism that thrives in extreme environments, environments in which humans and most other forms of life could not survive. o TYPES OF EXTREMOPHILES ACIDOPHILES ALKALIPHILES HALOPHILES HYPOTHERMOPHILES PIEZOPHILES CRYOPHILES IRREVERSIBLE INHIBITION A competitive enzyme inhibitor decreases enzyme activity by binding to the same active site as the substrate Binds reversibly to an enzyme active site and the inhibitor remains unchanged (no reaction occurs) The enzyme – inhibitor complex formation via weak interactions (hydrogen bonds, etc.) Competitive inhibition can be reduced by simply increasing the concentration of the substrate A noncompetitive enzyme inhibitor decreases enzyme activity by binding to a site on an enzyme other than the active site Causes change in the structure of the enzyme and prevents enzyme activity Increasing the concentration of substrate does not completely overcome inhibition Ex. Heavy metal ions: Pb2+ , Ag+ , and Hg2+ An irreversible enzyme inhibitor inactivates enzymes by forming a strong covalent bon with the enzyme’s active site o The structure is not similar to enzyme’s normal substrate o The inhibitor bonds strongly and increasing substrate concentration does not reverse the inhibition process o Enzyme is permanently inactivated o E.g. chemical warfare agents (nerve gases) and organophosphate insecticides Optimal growth at pH levels of 3.0 or below Optimal growth at pH levels of 9.0 or above Salinity that exceeds 0..2 M NaCl needed for growth A temperature between 80˚C and 122˚C needed to thrive A high hydrostatic pressure needed for growth A temperature of 15˚C or lower needed for growth Extremozyme Application o Biotechnology industry – production of enzymes for industrial applications o Petroleum industry – oil well drilling operations o Environmental scavenging and removal of heavy metals o Environmental clean-up using genetically engineered extremophiles o Laundry detergents used in cold wash cycles ENZYME INHIBITION ENZYME INHIBITOR: a substance that slows down or stops the normal catalytic function of an enzyme by binding to it Two types of enzyme inhibitors: o Competitive inhibitors: compete with the substrate for the same active site Will have similar charge and shape REGULATION OF ENZYME ACTIVITY Enzyme activity is often regulated by the cell to conserve energy. If the cell rus out of chemical energy, it will die Cellular processes continually produces large amounts of an enzyme and plentiful amounts of products if the processes are not regulated General mechanisms involved in regulation: FEEDBACK CONTROL PROTEOLYTIC ENZYMES AND ZYMOGENS COVALENT MODIFICATION OF ENZYMES A process in which activation or inhibition of the first reaction in a reaction sequence is controlled by a product of the reaction sequence Regulators of a particular allosteric enzyme may be: → Products of entirely different pathways of reaction within the cell → Compounds produced outside the cell (hormones) Mechanism of regulation by production of enzymes in an inactive forms (zymogens) ZYMOGEN (pro-enzyme) – are “turned on” at the appropriate time and place → Example: (proteolytic enzymes) hydrolyze bonds in proteins A process in which enzyme activity is altered by covalently modifying the structure of the enzyme → Involves adding or removing group from an enzyme Most common covalent modification – addition and removal of phosphate group: → Phosphate group is often derived from an ATP molecule → Addition of the phosphate (phosphorylation) catalyzed by a kinase enzyme → Removal of the phosphate group (dephosphorylation) catalyzed by a phosphatase enzyme → Phosphate group is added to (or removed from) the R group of serine, tyrosine, or threonine amino acid residue in the enzyme regulated Allosteric Enzymes o Enzymes responsible for regulating cellular processes o Characteristics: All allosteric enzymes have quaternary structure Have two kinds of binding sites: for substrate (active site) and regulator (allosteric site) Active site – where the substrate binds lock-and-key Allosteric site “another site” – where the regulator binds; distorts active site Some regulators of allosteric enzyme function are inhibitors (negative regulators/allosterism), and some increase enzyme activity (positive regulators/allosterism). PRESCRIPTION DRUGS THAT INHIBIT ENZYME ACTIVITY Many common prescription drugs exert their mode of action by inhibiting enzymes Antibiotic: a substance that kills bacteria or inhibits its growth ANGIOTENSIN CONVERTING ENZYME (ACE Inhibitor) SULFA DRUGS Angiotensin II – is an octapeptide hormone that increases blood pressure via constriction of blood vessels ACE converts Angiotensin I to Angiotensin II in the blood ACE inhibitors block ACE reaction and thus reduce blood pressure → Lisinopril is an example of a ACE inhibitor Derivatives of sulfanilamide Sulfa drugs exhibit antimetabolite activities → Sulfanilamide is structurally similar to PABA (𝞺-aminobenzoic acid) which → → → bacteria need to produce coenzyme folic acid Sulfanilamide is a competitive inhibitor of enzymes responsible for converting PABA to folic acid in bacteria Folic acid deficiency retards bacterial growth and that eventually kills them Sulfa drugs don’t affect humans because we get folic acid pre-formed from food o PENICILLIN Bacteria have one structural feature not found in animal cells – a cell wall The bacterial cell wall precursor is a polymer of a repeating disaccharide unit with attached polypeptide side chains that end with a D-alanylD-alanine unit Transpeptidase catalyzed the formation of peptide cross links between polysaccharide strands in bacterial cell walls Penicillin acts by complexing with the enzyme transpeptidase that is responsible for cell wall synthesis Selective inhibits transpeptidase by covalent modification of serine residue The structural similarity between the penicillins and D-alanyl-D-alanine allows the antibiotic to act as inhibitory substrates for the transpeptidase enzyme Since animal cells do not have cell walls, there are no such enzymes to be affected and penicillin has no effect on animal cells MEDICAL USES OF ENZYMES Enzymes can be used to diagnose certain diseases o The appearance of these enzymes in the blood often indicates that there is tissue damage in an organ and that cellular contents are spilling out (leaking) into the bloodstream o Assays of abnormal enzyme activity in blood serum can be used to diagnose many disease states, some of which are listed in Table 21.3 o Enzymes can also be used in the treatment of diseases A recent advance in treating heart attacks is the use of tissue plasminogen activator (TPA), which activates the enzyme plasminogen When so activated, this enzyme dissolves blood clots in the heart and often provides immediate relief Medical use for enzymes is in clinical laboratory chemical analysis For example, no simple direct test for the measurement of urea in the blood is available. However, if the urea in the blood is converted to ammonia via the enzyme urease, the ammonia produced, which is easily measured, becomes an indicator of urea. This blood urea nitrogen (BUN) test is a common clinical laboratory procedure. High urea levels in the blood indicate kidney malfunction WATER-SOLUBLE VITAMINS: VITAMIN C GENERAL CHARACTERISTICS OF VITAMINS VITAMIN : an organic compound, essential in small amounts for the proper functioning of the human body, that must be obtained from dietary sources because the body cannot synthesize it. Must be obtained from dietary sources because human body can’t synthesize them in enough amounts Needed in micro and milligram quantities o 1 gram vitamin B is sufficient for 500,00 people Enough vitamin can be obtained from balanced diet Supplemental vitamins may be needed after illness Many enzymes contain vitamins as part of their structures – conjugated enzymes Two classes of vitamins o Water-soluble and fat-soluble Synthetic and natural vitamins have the same function Vitamin C, which has the simplest structure of the 13 vitamins, exists in two active forms in the human body: an oxidized form and a reduced form Humans, monkeys, apes, and guinea pigs need dietary vitamins Co-substrate in the formation of structural protein collagen o Collagen also contains hydroxylysine and hydroxylproline (important in binding collagen fibers together) o Hydroxylation of lysine and proline in collagen formation are catalyzed by enzymes that require ascorbic acid (Vit. C) and iron o In Vitamin C deficiency, hydroxylation is impaired, and the triple helix of the collagen is not assembled properly o Persons deprived from Vit. C develops scurvy, a disease whose symptoms include skin lesions, fragile blood vessels, loose teeth, and bleeding gums Functions as a general antioxidant for water-soluble substances in the blood and other body fluids. o Because of its antioxidant properties, vitamin C is often added to foods as a preservative o Beneficial for several other vitamins: active form of vitamin E is regenerated by vitamin C, and it also helps keep the active form of folate (a B vitamin) in its reduced state Involved in metabolism of certain amino acids Why is Vitamin C called ascorbic acid when there is no carboxyl group (acid group) present in its structure? o Vitamin C is a cyclic ester in which the carbon 1 carboxyl group has reacted with a carbon 4 hydroxyl group, forming the ring structure Riboflavin (Vitamin B2) WATER SOLUBLE VITAMINS: THE B VITAMINS MAJOR FUNCTION: B Vitamins are components of many coenzymes Serves as temporary carriers of atoms or functional groups in redox and group transfer reactions associated with metabolism Niacin (Vitamin B3) Pantothenic acid (Vitamin B5) Thiamin (Vitamin B1) “Free” thiamin’s structure consists of a central carbon atom to which is attached a six-membered heterocyclic amine and a five-membered thiazole (sulfur-nitrogen) ring system The name thiamin comes from “thio,” which means “sulfur” and “amine” which refers to the numerous amine groups present The coenzyme form of thiamin is called thiamin pyrophosphate (TPP), a molecule in which a diphosphate group has been attached to the side chain The coenzyme TPP functions in the decarboxylation of a-keto acids Vitamin B6 (Pyridoxine, Pyridoxal, and Pyridoxamine) Biotin (Vitamin B7) Riboflavin’s structure involves three fused sixmembered rings (two of which contain nitrogen) with the monosaccharide ribose attached to the middle ring Riboflavin was once called the “yellow vitamin” because of its color Its name comes from its color (flavin means “yellow” in Latin) and its ribose component. Two important riboflavin-based coenzymes exist: → flavin adenine dinucleotide (FAD) → flavin mononucleotide (FMN). Both coenzymes are involved with oxidation-reduction reactions in which hydrogen atoms are transferred from one molecule to another. Niacin occurs in food in two different, but similar, forms: nicotinic acid and nicotinamide It was prepared by oxidizing nicotine using nitric acid; hence the name nicotinic acid When the biological significance of nicotinic acid was realized, the name niacin was coined to disassociate this vitamin from the name nicotine and to avoid the perception that niacin-rich foods contain nicotine or that cigarettes contain vitamins The name niacin is derived in the following manner The name pantothenic acid is based on the Greek word “pantothen,” which means “from everywhere.” This vitamin is found in almost every plant and animal tissue. Pantothenic acid-containing coenzyme o Coenzyme A (CoA) → one of the most used of all vitamin B coenzymes, contains pantothenic acid as part of its structure → required in the metabolism of carbohydrates, lipids, and proteins, where it is involved in the transfer of acetyl groups between molecules o Acyl Carrier Protein (ACP) → a “giant coenzyme A molecule.” → important in the biosynthesis of fatty acids Collective term for three related compounds: o pyridoxine (found in foods of plant origin) o pyridoxal and pyridoxamine (found in foods of animal origin) Contain an added phosphate group, are related to each other in the same manner that the “free” forms are related to each other Coenzymes participate in reactions where amino groups are transferred between molecules. Such transfer occurs repeatedly when protein molecules are metabolized Unique among the B vitamins in that it can be obtained both from dietary intake and also via biotin-producing Folate (Vitamin B9) Vitamin B12 (Cobalamin) bacteria (microbiota, hence the name biotin) present in the human large intestine Fused two-ring system with one ring containing sulfur and the other ring containing nitrogen o Attached to the sulfur-containing ring is a pentanoic acid residue “Free” biotin is biologically active The coenzyme form of biotin is formed by the carboxyl group of biotin’s pentanoic acid attachment forming an amide linkage with a residue of the amino acid lysine present at the enzyme’s active site As a coenzyme, biotin is a carrier for CO2; it has a specific site (a nitrogen atom) where a CO2 molecule can become attached. Several forms of folate are found in foods. All of them have structures that consist of three parts: 1. a nitrogen-containing double-ring system (pteridine) 2. para-aminobenzoic acid (PABA) 3. one or more residues of the amino acid glutamate. Folic acid – folate when only one glutamate residue is present Polyglutamates – folate molecules that have three or more glutamate residues Tetrahydrofolate (THF) o Active coenzyme form of folate o Only one glutamate and four hydrogen atoms have been added to the double-ring nitrogen system o Needed in methylation reaction (one or more methyl groups are transferred from one molecule to another) Folate o comes from the Latin word “folium,” which means “leaf” o Dark green leafy vegetables are the best natural source for folate COBALAMIN o comes from the fact that an atom of the metal cobalt and numerous amine groups are present in the structure of vitamin B12, which is by far the most complex of all vitamin structures unique in that it is the only vitamin that contains a metal atom “free” vitamin B12 and coenzyme vitamin B12 difference o Cyanocobalamin – free form o Methylcobalamin – coenzyme form only microorganisms can produce it; it cannot be made by plants, animals, birds, or humans. FAT-SOLUBLE VITAMINS VITAMIN A, D, E, K o Involved in plasma membrane processes o More hydrocarbon like with fewer functional groups o Occur in the lipid fractions of their sources o Their molecules have double bonds or phenol rings, so oxidizing agents readily attack them o Destroyed by prolonged exposures to air or to the organic peroxides that develop in fats and oils turning rancid o Because the fat-soluble vitamins are easily oxidized, they destroy oxidizing agents (which are involved in the development of coronary heart disease, genetic mutations, and cancer) VITAMIN A o Primary alcohol of molecular formula C20H30O; occur only in the animal world, where the best sources are cod-liver oil and other fish-liver oils, animal liver and dairy products o Provitamin A – is found in the plant world in the form of carotenes. Provitamins have no vitamin activity; however, after ingestion in the diet, β-carotene is cleaved at the central carbon-carbon double bond to give 2 molecules of Vitamin A o VISION: in the eye- vitamin A combines with opsin protein to form the visual pigment rhodopsin which further converts light energy into nerve impulses that are not sent to the brain REGULATING CELL DIFFERENCE: a process in which immature cells change to specialized cells with function Ex: differentiation of bone marrow cells white blood cells and red blood cells MAINTENANCE of the health of epithelial tissues via epithelial tissue differentiation Lack of vitamin A causes skin surface to become drier and harder than normal REPRODUCTION AND GROWTH: in men, Vitamin A participates in sperm development. In Women, normal fetal development during pregnancy require vitamin A. o o o o VITAMIN D – SUNSHINE VITAMIN o The antirachitic vitamin o Necessary for the normal calification of bone tissue o It controls correct ration of Ca and P for bone mineralization o Two forms active in the body: Vitamin D2 (ergocalciferol) and D3 (cholecalciferol) o o o VITAMIN K – ANTIHEMORRHAGIC VITAMIN Pigment in the skin, 7-dehydrocholesterol, is a provitamin D; when irradiated by the sun becomes converted to Vitamin D3 Humans exposed to sunlight year-round do not require dietary Vit. D Vitamin D3 (cholecalciferol) is sometimes called the “sunshine vitamin” because of its synthesis in the skin by sunlight irradiation o o A most important location in the human body where vitamin E exerts its antioxidant effect is the lungs, where exposure of cells to oxygen (and air pollutants) is greatest. VITAMIN E – ANTISTERILITY VITAMIN o Alpha-tocopherol – is the most active biological active form of Vitamin E o Tocopherol came from the greek word meaning, promoter of childbirth o Functions in the body as an antioxidant in that it inhibits the oxidation of unsaturated fatty acids by O2 o Primary function: Antioxidant – protects against oxidation of another compounds o There are four forms of vitamin E: alpha-, beta-, delta-, and gamma-tocopherol. These forms differ from each other structurally according to which substituents (9CH3 or 9H) are present at two positions on an aromatic ring. o The tocopherol form with the greatest biochemical activity is alpha-tocopherol, the vitamin E form in which methyl groups are present at both the R and R9 positions on the aromatic ring. Gamma-tocopherol is the main form of vitamin E in vitamin-E rich foods. o o o o Vitamin K1 also called as phylloquinone, has a side chain that is predominantly saturated; only one carbon–carbon double bond is present (found in plants) Vitamin K2 also called as menaquinones, with the various forms differing in the length of the side chain (found in animals and huans and can be synthesized by bacteria) Synthesized by bacteria that grow in colon Active in the formation of proteins involved in regulating blood clotting Deficiency may occur during the first few days after birth, because newborns lack the intestinal bacteria that produce Vitamin K and because they have no store of Vitamin K(it does not cross the placenta) Deficiency may also occur following antibiotic therapy that sterilizes the gut ENZYME KINETICS