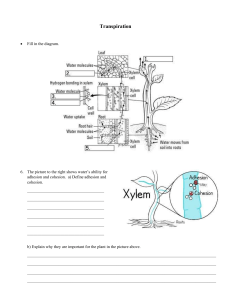
Section 2.1 - The Role of Water Cycles in Matter Key terms: biogeochemical cycles, hydrologic cycle, polar, hydrogen bond, cohesion, adhesion Terms/Focusing Questions Content What elements are necessary to life? Water, oxygen, carbon, sulfur, nitrogen, phosphorous BIOGEOCHEMICAL CYCLES Bio - life/living, geo - of Earth, chemical - relevant elements, cycle circular path Def. The routes that water and other chemical nutrients take through the biotic and abiotic components of the biosphere. Biosphere - all the areas on Earth (in the air, land, and water) that are inhabited by and that support life Can you give an example of how one of the elements above can travel great distances? HYDROLOGIC CYCLE The name of the cycle for WATER is the hydrologic cycle. (pg. 35) Walking water demo Describe the processes involved in the hydrologic cycle in a brief paragraph, beginning and ending with water storage in the atmosphere. Water is stored in the atmosphere in all three phases. When water vapour condenses, water falls as precipitation as rain and snow. Precipitation may be stored as ice or snow, infiltrate the ground to be stored as groundwater, or enter fresh/saltwater bodies. Water stored as ice or snow eventually melts and runs off into ground or surface water bodies. Plants take up water from the soil and return it to the atmosphere via transpiration. (Animals also take up water and lose it to the atmosphere.) Water evaporates from the soil, oceans, and other water bodies. Following these various pathways, water returns to the atmosphere for storage, completing the cycle. What are some unique properties of water? ● Universal solvent (A) ● Relatively high boiling and melting points (B) ● Adhesive and cohesive properties (C) ● High heat capacities (D) Video - How polarity makes water behave strangely (A) Water as a universal solvent. DO ON YOUR OWN (pg. 35-36) What is a UNIVERSAL SOLVENT? A solvent is a substance that dissolves other substances into itself - it does the action of dissolving. Universal means that it dissolves almost every substance in the biosphere, good and bad … POLARITY What property of water makes it a UNIVERSAL SOLVENT? Describe this property. Water is polar, like a magnet with N and S poles. This polarity allows it to dissolve substances. Polarity of water demo Salt dissolving Draw: HYDROGEN BONDS Explain why water is POLAR. Water is a molecule of one oxygen atom and two hydrogen atoms in a “V” shape. Since the hydrogens are cations - or positively charged within the molecule - and since they are on the same side, they create a positive pole. The oxygen is anion - or negatively charged - forming a negative pole. What type of BOND does water’s POLARITY create between other water molecules or solutes? Describe this BOND in terms of how water dissolves solutes. Water’s polarity allows for the formation of hydrogen bonds. This is a weak bond between hydrogen of one molecule and oxygen of another molecule (or negative ion). This bond pulls solutes apart into their elemental ions; this is the action of dissolving. (B) Water has a relatively high boiling point and melting point. Water freezing Why does ice float? TED-ED Ice floats Explain how the HYDROGEN BOND allows water to stay as a LIQUID over a large temperature range. As a liquid, the hydrogen bonds form, break, and reform frequently. This requires more energy input to break many bonds to boil water. Thus, molecules in water are packed together quite closely in this phase. They continue to pack together closer as the temperature decreases until water reaches its greatest density (1kg/L) at 4 °C. Explain how the HYDROGEN BOND allows ice to float. Unlike the solid and liquid states of most other substances, ice is less dense than liquid water. When water freezes, it expands because hydrogen bonds hold the water molecules in an open crystal structure. This prevents the water molecules in ice from getting as close to each other as they do in liquid water. This expansion begins as the temperature decreases below 4 °C. How does hydrogen bonding affect nutrient cycling? (C) Water has adhesive and cohesive properties ADHESION COHESION How do adhesion/cohesion affect transpiration? Explain the role of HYDROGEN BONDING in: ● Transporting nutrients over a large temperature range: Universal solvent: dissolves nutrients High specific heat capacity: constant internal temperature for animals that have a high concentration of water in its tissues ● Nutrient cycling in lakes: Universal solvent: dissolves nutrients Density: changes in density with changes in temperature allow for higher density water to sink and be replaced by lower density water Define ADHESION: attraction to other molecules Define COHESION: attraction between water molecules Explain the role of ADHESION and COHESION in: ● Surface tension: the attraction of water molecules to each other allows for many insects to walk on water as well as keep organic debris on the water surface where it provides nutrients for aquatic organisms ● Transpiration: The attraction to both the walls of the xylem (adhesion) and each other (cohesion) allows water to counteract gravity as it moves up the xylem to replace water that has evaporated from the leaves via transpiration. Beaker-yarn demo (D) Water has a high heat capacity How does water’s high heat capacity benefit the biosphere? Define HEAT CAPACITY: measure of the amount of heat energy a substance can absorb or release for a given change in temperature ○ ○ ○ How do organisms gain and lose water? Individual level: Allows life to maintain a fairly constant internal temperature Ecosystem level: Oceans and lakes moderate the air temperature near land Global level: Surface currents distribute heat from warm equatorial regions to colder latitudes Gain water Lose water eating breathing drinking sweating absorption (through skin) Waste (urine and feces) metabolic water What is metabolic water? Metabolic water is the water created through cellular processes (metabolism). Summary: biogeochemical cycles allow nutrients that are key to life to cycle throughout the globe; water is an example of one of those important nutrients (hydrologic cycle); water is an incredibly significant nutrient for living things due to 4 main characteristics: universal solvent, high boiling/melting points, cohesion and adhesion, and high heat capacity Crash Course Hydrologic Cycle (0-5:13) DO: Thought Lab 2.1 - Water Gains and Losses Video - Droughts in the Canadian Prairies Why is water important? Define Water Quality: how inundated water is with toxic chemicals or pathogens Water quality is important for: ● Society: ● Ecosystems: Do: Section 2.1 Review, pg. 40 Q 4, 5, 7, 8