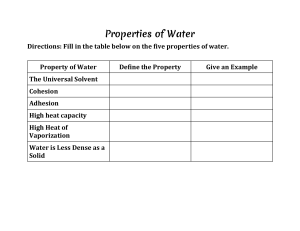
Ms. Goolsarran Name ______________________________________ Period __________ Questions/Notes for Biology QUESTIONS ANSWERS Water Molecule-40-41 1. Indicate all of the following on the water molecule a. Oxygen molecule b. Hydrogen molecules c. Negative charge d. Positive charge 2. a. How many oxygen atoms are in a water molecule : Answers a. b. How many hydrogen atoms are in a water molecule b. c. Which atom is the larger one? c. 3. A molecule that has unevenly distributed charges is said to be_____________________ Answer a. 4. a.Take two water molecules and bring them close together. The hydrogen of one water molecule is attracted to what part of the other water molecule? Answer a. Date _______________ b. What type of bonding is this? b. 4. Indicate on the diagram using dashed lines (----) where hydrogen bonding would exist 5. What causes water to expand when it is forming into ice? Answer a. 6. Circle the letter of each sentence that is true about water's cohesion. Circle a. It helps some insects can walk across the surface of the water. b. It refers to the tightness or smoothness across the surface of the water. c. It is caused by water molecules not being attracted to each other. d. It causes water to form round beads. 7. Use the following words in an accurate sentence about cohesion Answer Attraction, molecules, water, cohesion 8. Choose one part of the water properties lab to explain how you saw cohesion a. Water on the dime b. Paper clip floating on the surface of water c. Water forming beads on the wax paper d. Black pepper floating on water Explanation Your answer must include key words about cohesion 9. Circle all the true statements about adhesion Circle a. it is an attraction between molecules of different substances b. it causes water to rise when in a glass cylinder c. it causes capillary action in plants 10. Choose one part of the water properties lab to explain how you saw adhesion or not at work a. Water and the paper towel b. Water in the glass graduated cylinder c. Water not forming beads on the wax paper d.it can happen between water and oil molecules Explanation Your answer must include key words about adhesion 11. It is a rainy day, explain how you would see a. Cohesion because of the rain b. Adhesion because of the rain Explanations a. b. 12. Fill in the blanks with the following words Heat capacity of water is high because it takes a lot of Hydrogen bonds, heat, faster, increase, temperature ___________ energy for the molecules to move ____________, this raises the _______________ of the water. Water’s head capacity, is the amount of heat require to ______________ the temperature. Solutions and Suspensions p.42 13. a. True or False- a mixture can be chemically combined a.(Circle). True b.Give two examples of mixtures you can make in your kitchen using water Two examples b. c, False 14. a. Definition of solvent. Choose an answer by circling the letter 15. Identify the solute and solvent in the following situations Example: salt (solute, solvent) and water (solute, solvent) a. The substance being dissolved b. The substance that is doing the dissolving a. Dishwashing soap (solute, solvent) + water (solute, solvent) Nail polish(solute, solvent) + nail polish remover (solute, solvent) Air bubbles (solute, solvent) + soda (solute, solvent) b. c. 16. Why is blood considered a suspension? ACIDS and BASES pg. 43-44 17. a. a.When water splits, what are the two ions that it splits into? b.Which one is negative? Which one is positive? b. 18. Which ion does the pH scale indicate? Circle the choice a. H+ b. OH- Acids, Bases, and pH 19. Circle all choices that are true of acids 20. Circle all choices that are true of bases a. Circle Acids have more H+ than OH- b. Acids are found above 7 on the pH scale c. Acids can be found in some of the foods we eat d. a. Acids tend to taste sour Circle Bases have more OH- ions than H+ b. Bases are above 7 on the pH scale c. Soap is an example of base d. Lemon juice is a base Answer: 21. During exercise, many chemical changes occur which causes a drop in pH, which can be very serious. How does the body deal with changes in pH? HOMEWORK