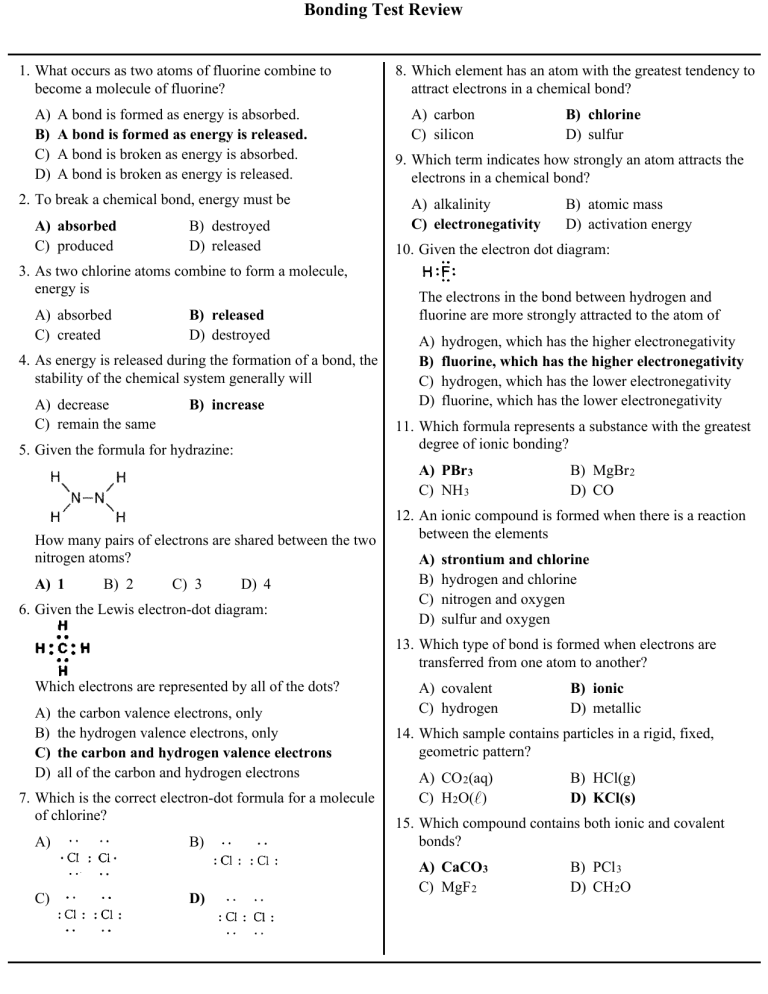
Bonding Test Review 1. What occurs as two atoms of fluorine combine to become a molecule of fluorine? A) B) C) D) A bond is formed as energy is absorbed. A bond is formed as energy is released. A bond is broken as energy is absorbed. A bond is broken as energy is released. 2. To break a chemical bond, energy must be A) absorbed C) produced B) destroyed D) released 3. As two chlorine atoms combine to form a molecule, energy is A) absorbed C) created B) released D) destroyed 4. As energy is released during the formation of a bond, the stability of the chemical system generally will A) decrease C) remain the same B) increase 8. Which element has an atom with the greatest tendency to attract electrons in a chemical bond? A) carbon C) silicon 9. Which term indicates how strongly an atom attracts the electrons in a chemical bond? A) alkalinity C) electronegativity 10. Given the electron dot diagram: The electrons in the bond between hydrogen and fluorine are more strongly attracted to the atom of A) B) C) D) hydrogen, which has the higher electronegativity fluorine, which has the higher electronegativity hydrogen, which has the lower electronegativity fluorine, which has the lower electronegativity A) PBr 3 C) NH 3 How many pairs of electrons are shared between the two nitrogen atoms? B) 2 C) 3 B) atomic mass D) activation energy 11. Which formula represents a substance with the greatest degree of ionic bonding? 5. Given the formula for hydrazine: A) 1 B) chlorine D) sulfur D) 4 6. Given the Lewis electron-dot diagram: B) MgBr 2 D) CO 12. An ionic compound is formed when there is a reaction between the elements A) B) C) D) strontium and chlorine hydrogen and chlorine nitrogen and oxygen sulfur and oxygen 13. Which type of bond is formed when electrons are transferred from one atom to another? Which electrons are represented by all of the dots? A) B) C) D) the carbon valence electrons, only the hydrogen valence electrons, only the carbon and hydrogen valence electrons all of the carbon and hydrogen electrons 7. Which is the correct electron-dot formula for a molecule of chlorine? A) C) B) D) A) covalent C) hydrogen B) ionic D) metallic 14. Which sample contains particles in a rigid, fixed, geometric pattern? A) CO 2(aq) C) H2O( ) B) HCl(g) D) KCl(s) 15. Which compound contains both ionic and covalent bonds? A) CaCO3 C) MgF 2 B) PCl 3 D) CH 2O Bonding Test Review 16. Which of the following solids has the highest melting point? A) H2O(s) C) SO 2(s) B) Na2O(s) D) CO 2(s) 17. What is the total number of electron pairs shared between the two atoms in an O 2 molecule? A) 1 B) 2 C) 6 D) 4 18. A molecular compound is formed when a chemical reaction occurs between atoms of A) B) C) D) chlorine and sodium chlorine and yttrium oxygen and hydrogen oxygen and magnesium 19. Which characteristic is a property of molecular substances? A) B) C) D) good heat conductivity good electrical conductivity low melting point high melting point 20. Which type of substance is soft, has a low melting point, and is a poor conductor of heat and electricity? A) network solid C) metallic solid B) molecular solid D) ionic solid 21. Magnesium nitrate contains chemical bonds that are A) B) C) D) covalent, only ionic, only both covalent and ionic neither covalent nor ionic 22. Base your answer to the following question on Which statement correctly describes the bonds in the electron dot formula shown to the right? A) One of the bonds represented is ionic B) All of the bonds represented are ionic C) One of the bonds represented is coordinate covalent D) All of the bonds represented are coordinate covalent 23. A solid substance is an excellent conductor of electricity. The chemical bonds in this substance are most likely A) ionic, because the valence electrons are shared between atoms B) ionic, because the valence electrons are mobile C) metallic, because the valence electrons are stationary D) metallic, because the valence electrons are mobile 24. Which substance contains metallic bonds? A) Hg( ) C) NaCl(s) B) H2O( ) D) C6H12O6(s) 25. Silicon dioxide (SiO 2) and diamonds are best described as A) molecular substances with coordinate covalent bonding B) molecular substances with ionic bonding C) network solids with covalent bonding D) network solids with ionic bonding 26. Which molecule has a nonpolar covalent bond? A) B) C) D) 27. Which compound has hydrogen bonding between its molecules? A) CH 4 B) CaH 2 C) KH D) NH 3 28. Which formula represents a nonpolar molecule? A) CH 4 B) HCl C) H2O D) NH 3 29. Which statement explains why a molecule of CH 4 is nonpolar? A) The bonds between the atoms in a CH4 molecule are polar. B) The bonds between the atoms in a CH4 molecule are ionic. C) The geometric shape of a CH 4 molecule distributes the charges symmetrically. D) The geometric shape of a CH4 molecule distributes the charges asymmetrically. Bonding Test Review 30. Hydrogen bonding is a type of A) B) C) D) strong covalent bond weak ionic bond strong intermolecular force weak intermolecular force 31. Which type of attraction results from the formation of weak momentary dipoles? A) B) C) D) ionic metallic molecule-ion van der Waals forces 32. The table below shows the normal boiling point of four compounds. 33. When calcium chloride is dissolved in water, to which end of the adjacent water molecules will a calcium ion be attracted? A) B) C) D) 34. Which diagram best illustrates the ion-molecule attractions that occur when the ions of NaCl(s) are added to water? A) B) C) D) Which compound has the strongest intermolecular forces? A) HF( ) C) CH 3F( ) B) CH 3Cl( ) D) HCl( ) the oxygen end, which is the negative pole the oxygen end, which is the positive pole the hydrogen end, which is the negative pole the hydrogen end, which is the positive pole Answer Key Bonding Test Review 1. B 2. A 3. B 4. B 5. A 6. C 7. D 8. B 9. C 10. B 11. A 12. A 13. B 14. D 15. A 16. B 17. B 18. C 19. C 20. B 21. C 22. C 23. D 24. A 25. C 26. A 27. D 28. A 29. C 30. C 31. D 32. A 33. A 34. A

