C.t"'.is+or*. yu-Bq.Jt
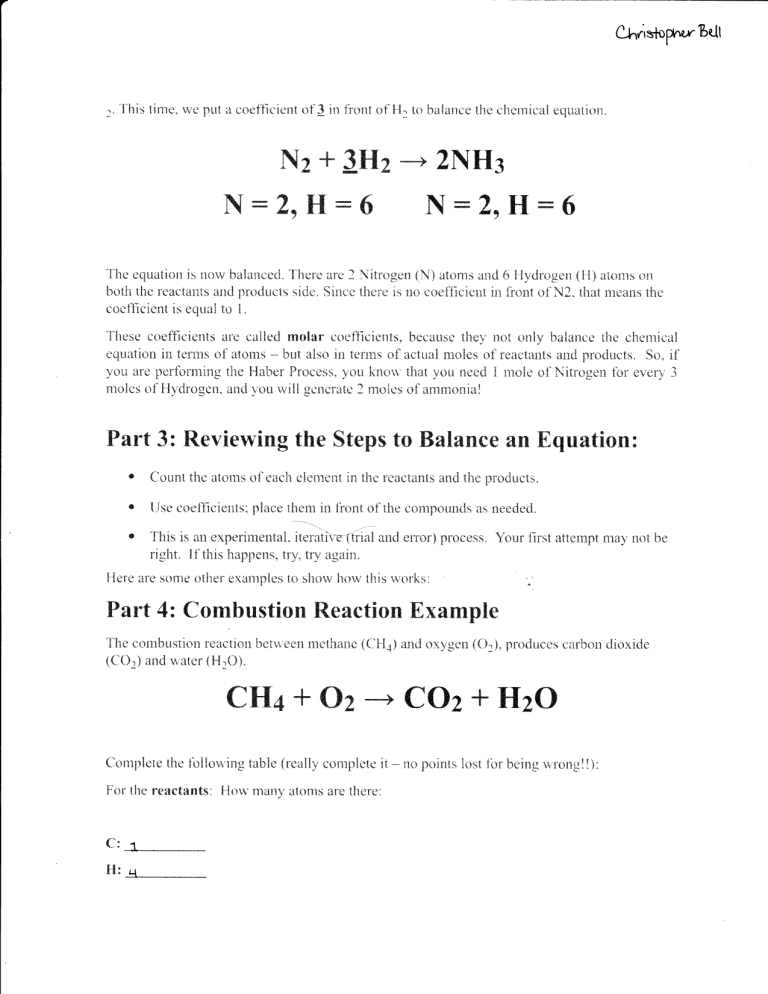
,. '['his time, we put a e*et]icient of 3 in fr*nt nf ]1. tc balance lhe
cllerr"]i{ral equation"
ffix+ffi$#x*&trrus#g
ruffiffi*&sffiffi
XHffiXu
&tr
ffi
ffi
s
'ille equatitin is norv halanced. There
are 3 Nifr*gerl {.i\) atom$ and 6 }{ydl:rige* (li} atorn:i on
hoth the rsactailt$ and praduels side. Since th*re ix r* cqret{irieut in lir:nt of N2. that means the
coellcient is equal to L
These coefficients are call*d mclrr caefficis*ts, because th*y nct only balancs the chemical
equation in tenus of *toms -* but also il tefil:s *f actual moles of reactarts and products. So, if
you are perlonning rhe Haher Procrss, you kn*r,v that you neert I rxr:le of Nitr*gen for every 3
mol*s of Hydragen. and ),ou rvill gencratc ? mai*s of arnmoxisl
Part 3: Reviewing the Steps to Balance an Hquation:
.
r
r
Count the atrms of each *lemeut in the r$acra^nts and the products.
[Jse eoefficie:lts; piace theni in froni o1'the c*mpounds as neaded.
This is an experirnental, iteralieldal and enor) flroeess. Your first attempt may n*t be
right. If this happens, try, try agilin.
Here are sorne *ti:er examples to shew hr:w fhis n
orks:
;.
Part 4: Combustion Reaction Example
The combu*tior, ,*u"ti,rn bet,"r'een mcthane {C:H4} and oxygen {O:}, pr*rduces carbon dioxiiie
(CO:) and water {H:O}.
tr$S* &
ffix
Complete the lbllawing table {real}y cor:nplet* it
Fi:r the
ff:1
$^$l r{
re*ctants:
t-it";r,l'many atcms are there:
trffie + SSxffi
*
no p*irtts lost fbr being wrongl!)
{}I
2-
I'*r-
f1:*:
["$:
*L
pr*c*t*c:tg; i {{,}bv
{};3**__
.
r"l}Ltn\., fi[*m"]s
i]rf
tilt*t-*
{"iql tr: {E-:* rl**xt p;rg;* l*r:ct
._.-
trffiS* & ffix *-p
F*r" xi:* r*m*fm$&ts. fl:e.r*
t
trffix *
*r* if vetr".r.r"r
right,
SSxffi
ilr*,
x
r$
d
{}
tu
,?
Fr:r th* g:r*rItt*f$* t]:*t-* (rr*;
.fi-fl
?
.,&,
ffi;-J-
Nexl. to staff balancing the arom$, try balancing the Carbon {C} atr:*rs timt, fhen the Hydrogen
(l{} atoms, and then thi: Oxyge:: {O} atCIrns.
ln this case. the carbon {C) atoms are already }ralanced. So nrw we bok tt the hydrogen (FI}
tttoms. There are 4lrydrogen iH) Bl*m$ cn the reactarts side and 2 hydrogen (H) atorns on the
products sittrc. To balaacc thr:m. we put a coetllsicnt of-2 il flr*nt *f I-{.:0.
CH*+Oe-lCO1*SHzO
i;q:e"
th* a'*ffifl€a,*$?*t$" tfl:*s"* ;tflt
Fr*r"
tlt*
$sr#*{re $$. t}:*l
* itr*;
I
Now that the *xygerr i]I{}ry}s are halanced, we naed t* balanr:e lhe iron {Fe} ;*tams tirst. To do this"
we need to put a r:ce11-i*ierrt of 4 in frout trl Fe i* tire pr*ducts sid*. n*ou,that th* F'e atorns are
halanced, \,ve cah lralance the carb*n atoms. Wc clCI this by ptrtting r ccef{icienr of ^i in {iont of Cl
on th$ reactants side. 'fhe cir*r*ical equation is nqlu. b&lafie*,iJ.
ffiffi=ffimffim
+ ffitr *h
,*
*i=#:
ry,
{};
flro
\".
3
c
& Strffix
ffiS=*
f.*
*B
{--;
$
Part 6: More Counting Atoms and thinking deeper
Two balanced chemical reactions {or chemical equations) are given below
Ii
"
Solid, g : Gas):
Cu{*} + H:* {g}
{g}---+ }C*: {g}
CUS {s} + H3 {g}
?) 2 CO {g} * *:
I
{s:
---+
Indicate th* r*aclants ar.:d
R*a*ta:rt{ s}
f*r *a*h r*acti** in t}:s table belfiw:
Pr*rJu*t{*}
Rea*ti+;n
H ("ryP-"zcn{3r.n, \c!'s5f;} ].C*l , 1' O,2f\
LLrrtO
2 L, B O LU-r,r"", L*4dvs3.,^)
1 Cu,
1)
2)
t
O, L
2" What d*es the arr*rr, r-spr***llt
Tlt
ayy$rro rognrsrrrts -Ifu-trartrsthun
in a ch*r*i*al reacdi*tt?
So^
rLq^r+ornllta
{ro pru*rx;rt
3. For reaction (l), how many H atoms, Cu atoms, and O atoms are represented on:
a) the r*acta*t side?
2-A
1-
c,
1-O
b) the product side? 2H, 1Co, l"0
4. For reaction (2), how mary C atoms and O atoms are rspresented on:
a) thc rcactant sid*l?
ZL
AtO
b) thc prsduct sidr?
ZL
l-t D
5.
Based on your allswers to questions 3 and 4, what general statement can be made about
the number of atoms of each type on the two sides of a chemical equation? Describe
yom answer in the space provided.
V(!- (r'n torrct".rd,c 4h^* rt"rt rsu'*{S" *t^"rui",.g eqot;[o^{o br' bolarurd o'vrd -{r,o}
'l+.L &.vn1, qsa^ti\ oP atuqs fU+ 3,',tUrf"u rtariron wr,uet br- Pr&\u'!*'yr {rr. ?*do+'
ffimrf ?x ffme*ffef*xxg &#qx#**xx*ms
ffitre#
&,'*qxXmm
A balanced chemical reaction can be interpreted in two ways. First, it can'te thought of as
describing how many molecules of reactants are consumed in order to produce a certain number
of molecules of products. Analogously" it can be thought of as describing how maily moles of
reactants are consumed in order to produce the indicated number of moles of products.
Consider thc safiIe
tw* bala*ced r*acticn* frr:r* Part S:
i ) Cr:O {s} + H3 {.9} -+ Cra{s} + H3* {e}
:) 2 CO ts) * O: {s} --} }C*a te}
L
How many H2O molecules are produced for every H2 molecule that is consumed in
reaction (l)?
Orrr- HXO rwotr.qotr-'," F,"U*fa t>" wer\ H1
2.
Far r$ection {2}:
vrorCwU- -l'tno"t iS Lu^s,nrcl\{a-tlnr,vtnurrsr,r
a)
How many CO2 molecules are produced for every 02 molecule consumed?
Rr e,u\ 02 nnolr"rotr- to,r*snad z onr- LO2 mo\r,r.r.itr 16 plr ;tr^t
b)
How many CO2 molecules are pro&tced for every CO molecule consumed?
OvrU CO2 ryls\rrd\r-'\\ p/Dd"r.rd & *1^bO1 vwoVr*w 6^rs\t\,\tu[
c)
How many molecules of CO2 are produced when 2 molecules of 02 are consumed?
T,rro mc\^rreki uF COL A'rL P/'Od'Id r^rr,rr A n'\!\r-rrUl al O" oqrL c,D/usoflrd
d) How many moles of CO2 are produced
Trrn rng\r-r
ol CO" arupodo,rA t r^u\ 5
when 5 moles of 02 are consumed?
wlotrA oP Dz 6vL c,o*omrd
3.
How many moles of CUO react in order to produce 12 moles of Cu in reaction (l)?
Tl-"I*, rvrolt-s of CoO re,l'db t'& P6{.r*to po'ur- l?- wrotr-s oC C'tr
4.
Determine the number of reactant molecules and the number of product molecules for
reaction (l) and reaction (2).
1 - Two rvrotuu\rs iv't -llnr- ngc+o'd's; 'lun rnctr,r-:Us irt+t'cpro'Io<,le
L * S-. rrrro\riJu-r in-{dr
5. Is the number
reactions?
il;;t-
of molecules identical on the reacknt and product sides of these balanced
rn iurrr-u is *g| iar".ts,"rQ on poAl srdr> oP'{'lnr t- '"-g"rt <1. v\arrtie',\,
-lilnc nrn&r.rn 6p6Jstt'r:,
Does the total numbcr of moles of gas increase, decreasc, or remain constant when
reaction (2) occurs?
&'rfuory't
o,F morr+ sS
l"i,
"e,o,,h"" 2-,
"6"
^unUrr
7.
"ta
Explain how your answers to the previous question can be consistent with the idea that
atoms are neither created nor destroyed when chemical reactions take place.
l*,U,,' fo+ {1xerJ|rr*
^.nn:urct
d,"m^n
8.
t D Yffr&d\{5in-lht-P^ttd
o,F
l.rq,,,lc rera
6.
yer-&*crrts;
,.ro,rwon- eS
rnlr-r oft- @nv,^r{-d tn &|rrr4rho^zblrl{hco,irvrr
# "*r^. a.ft- pfirtrr * ^"6'a*^x
"Sfha,
vtrxr'ror-r oP
vtt"rrs'
Ttt *
$
cto*^wttO,
r,tty
Is it correct to state that if 100 grams of CuO are consumed whsn rcaction (I) occurs. then
100 g of Cu are formed inthe process? Why orwhy not?
No, Yoe ha,.X- fODtrE
v\S
d{,?ft;,r , -hru."r,{rrr, aUrrr,ir L'as {t'ran lOb 6ruwrs
[, *
pvr-
trbtrlaYru-
r,r*n
bd"ru*r 6PPt/*d
oppu inJhs (ot^+ ' 0+'drtL * tdld'
oF-{hLrvro\rsilc C,^lD uoVr,cn',s ais\"to.:tt-C
e"lrX
"f
9. Describe, using grammatically
correct English sentences, the steps taken to calculate the
number of grams of CO2 produced in reaction (2) given that X grams of O2 are
consumed.
Ne- undlxrto.rcl" -lhr- /oAo oD-ll^! rilp\r-u rn (,O2, $ tllarn thc arnoy*f ol lyorms uF Oz,
\^tL ca^ oruvoctc\ irtPerr bs{l^ S<, n su't of, C-oZ anrd C aloat'
Part 8r Counting Ch*rg*s
Atoms are neither created nor destroyed when chemical reactions take place
Therefore, the number of atoms of each element must be identical on the reactant (left) and
product (right) sides of a balanced chemical reaction. Such a chemical equation is said to be
atom-balanced.
By the same logic, protons and electrons ffie neither created nor destroyed whea chemical
reactions take place.
Therefore, the total charge must be identical on the reactant and product sides of a balanced
chemical reaction. Such a chemical equation is said to be charge-balanced.
In each of the balanced chemical reactions given below. the symbol "(aq)" indicates that the
molecule or ion is surrounded by water molecules.
I)
Ag*{aq} + tl-{aq}
2) zn{s} + cu}*{aq}
*-+
---+
AgCt{s}
zn?"tuq} + cu{s}
3) 3 CIO-{aq} --* ?CI-{aq}
+ ttr*}-{oq}
** 3C#*ioq} + Mg{s}
4)
?Cr:*{aq} * tuIg3*{uq}
l.
Confirm that each of the chemical equations in the above equations ue atom-balancedby
counting the number of atoms of each element on both sides of equation. Provide your
arrswers below.
I)
a+"un-oc..tqn
2)
+-{*"n-u.go^+r
rU (z - Z)
(z-4
3) oc* n+""n-r"olEr,^.c.or
(fo - 4{)
4) ++"nn- u"t."u d CS+)
2. a) For each of the chemical equations 14, determine
the sum of the charges on the lefthand side and the sum of the charges on the right-hand side" Provide your answers
below.
1) n, ^ rtrrc9
b\
21 _.tL--
3) -8..
._
_
4) +q
b) Based on these reactions, circle any, of the following statements
if correct.
.
The sum of the charges on both sides of a chemical equation must equal zero.
.
The sum of the charges on both sides of a chemical equation must be a positive number.
.
iii) The sum of
the charges on both sides of a chemical equation must be a negative
number.
No*.\fi\
3.
Provide a general statement that can be made to describe the sum of the charges on both
sides of a balanced chemical reaction.
Ji'.ct*^ #"!,o .J""*U. wa\r*+ bL aq*Q * b,oll^ Sdra u0 o- uo[r*r'-r-d c'{d'c'*[ rco'r*rar'
ffm*"f
1
S
: i&ffi$*ff$*m*$w
ffi
s
Write the balanced equation that expresses in chemical shorthand (atomic symbols) the
following statement:
"Iron rsacts with molccular oxygen {O2} to give iron oxide with thc formula Fe2O3"
FcO, +
F.g-+
FcxOg
-'*$il}.
)
7.. Llalaxr:e th* f$ll*llring
a"
reacfic*rls
Ca{OH}: + HCI --* CaCI* + HrO
-+
2-HU * C,*(oH)z
b.
Agf=f{}r + CaCl2
CaCt2 *
ZH2O
-* Ca{N*:}: +AgCl
ZA3Nob* CICJL .a Co(NDs)z * 2AtC\
s"
F*=O3 +
{l -* F* + CO:
F"eOs+[,
d.
e.
a
2f{-+
CD3
NaHC*: * H?S**
--+
ZNaHCOS+ HZSD'.
-?
C+Ht*
* Or * C*:
NarS*$
't-
H:O-+- CS:
NarSo1 + ZC,O.
r
ZHzD
+H=O
ZCrHro + lSOz = BC-Oz+ IDI{1O
f,
Me{*H}:
-r HEr
M3(Ort) L+ zHBr
g.
*SSBr:
* H:*
-r MSbz + 2H2$
Al"*3 * H=$*+ * AI3{S*+}: + H-,*
Atz0s+ SHzsDq
L
-*
+
KHC*3 + H3F*4
AtzGor).+ 3t-l10
---+
K:HFq3+
* H:C + COr
2Kt-tCo5+ H.Po, --? I(f-lPb.1 + ZF{zD +
i.
C*Hr** + *:
-r C*r + H?$
C-qH,oO+ lt Dr
A
QCAz+ St-'lzD
ZLor




