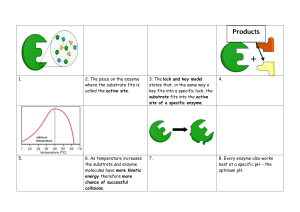
Biology 1401 Dr. Wedig What should I learn from Chapter 6? Define vocabulary/terms related to energy utilization and transformation. Explain the different sources of energy available to living organisms. Understand the process of energy transformation, and associated energy losses. Explain how enzymes function, and factors affecting rate of chemical reactions. Identify mechanisms involved in controlling chemical reactions (inhibition, activation, homeostasis) Bioenergetics Study of how organisms manage their energy resources Energy: fundamental to all metabolic processes Energy: capacity to do work Forms of Energy Kinetic energy: energy of motion Potential energy: stored energy Chemical energy: a form of potential energy energy is stored as bond energy Energy transformation: chemical energy can be transformed to kinetic energy Thermal Energy: heat Thermodynamics • Study of energy transformations • Laws of thermodynamics – Law of Conservation of Energy • Energy can be transferred and transformed, but can be neither created nor destroyed • Transfer or transformation of energy is not 100% efficient – some is released as heat – heat: energy of random molecular motion; most systems can not harvest this form of energy to do work – Law of Entropy Gasoline has potential energy in its chemical bonds Car converts the chemical energy of gasoline to kinetic energy Free Energy Portion of a systems energy which can perform work when temperature is uniform throughout the system Spontaneous reaction can occur without outside help increases stability of the system can be harnessed to do work Law of Entropy Every transfer or transformation of energy makes the universe more disordered Entropy: quantitative measure of disorder Life requires constant energy input Energy intake enables living systems to remain ordered Anything which interferes with energy intake alters ordered state (disease, injury) Transfer of energy in living systems is 30-40% efficient Complex organic molecules have great potential energy Types of Work Done by Cell • Mechanical work – contraction of muscle cells – beating of cilia – movement of chromosomes during cell division • Transport work – pumping substances across membrane • Chemical work – anabolic processes Coupled Reactions Part of a chain of events Exergonic reactions are coupled with endergonic reactions ATP hydrolysis (exergonic) is used to phosphorylate glucose (endergonic) Enzymes Proteins that serve as catalysts for chemical reactions taking place within living systems. Usually end with the suffix –ase Speeds up rate of reaction without being altered by the reaction Mechanism of Action: activation energy Does not change G Activation Energy (EA) Initial investment of energy required for a reaction to proceed Bonds of reactants break only when molecules have absorbed sufficient energy to become unstable Transition state: unstable form of molecule Heat: reaction rate by rate of molecular motion Active Site Region of enzyme that recognizes and binds substrate Enzymes are substrate-specific Specificity is based on three-dimensional shape (secondary, tertiary, quaternary structure) Only a few amino acids make up the active site (responsible for substrate specificity); rest of protein provides the framework that reinforces the configuration of the active site. Microenvironments Created by side chains of the amino acids at the active site Acidic conditions: transfer H+ to the substrate Basic: take H+ from the substrate May be brief covalent bond formed between enzyme and substrate to facilitate catalysis Models of Enzyme Function Involve formation of an enzyme-substrate complex Lock-and-key Active site is complementary to substrate (shape and functional groups) Induced fit Induced Fit As substrate enters active site, it induces the enzyme to slightly change shape Enzyme accommodates to substrate to bring chemical groups of active site into position to interact with substrate and enhance the ability of the enzyme to catalyze the reaction Hexokinase and Glucose Enzyme-Substrate Complex Substrate is held in place by ionic bonds, H bonds, and hydrophobic interactions A few amino acids dictate associations between enzyme and substrate Interactions between enzyme and substrate stabilize the transition state, thereby decreasing EA Chemical Energy and Life Exergonic reaction: net release of energy -G (change in free energy) characteristic of catabolic reactions Endergonic reaction: absorbs free energy from its surroundings +G characteristic of anabolic reactions G = Gfinal-Gstarting Redox Reactions Involve a transer of one or more electrons from one reactant to another reduction: gain of electrons (GER) oxidation: loss of electrons (LEO) Reducing agent: electron donor Oxidizing agent: electron acceptor Electronegativity: affinity for electrons Redox Reactions The Formation of Formaldehyde: A Redox Reaction Carbohydrates in Redox Reactions • C-H bonds store energy • Carbohydrates can act as electron donors in redox reactions – Produce energy in the form of ATP Factors Affecting Reaction Rate • Enzyme Concentration – enzyme concentration reaction rate – If enzyme concentration exceeds substrate concentration, adding more enzyme will not reaction rate • Substrate Concentration • Temperature • pH Substrate Concentration substrate concentration reaction rate until substrate concentration exceeds enzyme concentration Saturation: point at which all enzyme molecules are occupied Temperature Optimal temperature: fastest rate of reaction Reaction rate as temperature up to optimum Temperature optimum of most human enzymes is between 35 and 40 ° C Denaturation: destruction of H bonds due to excessive temperature Destroys secondary, tertiary, quaternary structure enzyme activity pH Optimum pH at which enzyme functions optimally Most enzymes: 6-8 Pepsin: 2 Trypsin: 9 Cofactors Nonprotein components required by an enzyme for its activity Inorganic/minerals Zn, Fe, Cu Organic/vitamins Control of Enzyme Activity Competitive Inhibition Allosteric Regulation Noncompetitive Inhibition Allosteric Activation Cooperativity Metabolic Control Noncompetitive Inhibitors Bind to enzyme at allosteric site Allosteric site: binding site separate from active site Binding of allosteric inhibitor changes the shape of enzyme and prevents it from binding substrate May be reversible or irreversible Can not be overcome by adding substrate Noncompetitive Inhibitors are also known as allosteric inhibitors. They bind to a site distant from or other than the active site. Competitive Inhibitors Bind to Active site Look like substrate; compete with substrate for active site Inhibition is reversible and can be overcome by adding substrate Penicillin blocks a bacterial enzyme that is involved in synthesizing the cell wall Competitive Inhibition: a portion of the inhibitor molecule looks like the substrate, and is recognized by the active site. Allosteric Activation An enzyme is inactive until the activator molecule binds to allosteric site Activator: stabilizes active site (active form of the enzyme) PFK and ATP/ADP levels Allosteric Regulation Allosteric Proteins often fluctuate between inactive and active forms. Allosteric activators and inhibitors Cooperativity Amplifies response One substrate molecule primes an enzyme to accept additional substrate molecules Hemoglobin and binding O2 Metabolic Control Each metabolic pathway (series of reactions that functions to convert a reactant(s) to an endproduct(s) is closely regulated by controlling enzyme activity Allosteric regulation PFK and ATP/ADP levels Feedback Inhibition: end-product of pathway feeds back and inhibits a regulatory enzyme Threonine isoleucine What did I learn from Chapter 6? Cells obtain energy needed to maintain life from chemical energy. These cells transform chemical bond energy to energy in the form of ATP; in the process of transformation, energy is lost as heat. Some important elements to energy extraction and ATP production by cells include: Enzyme function and factors affecting enzyme activity Feedback mechanisms, inhibitors, activators, coupled reactions