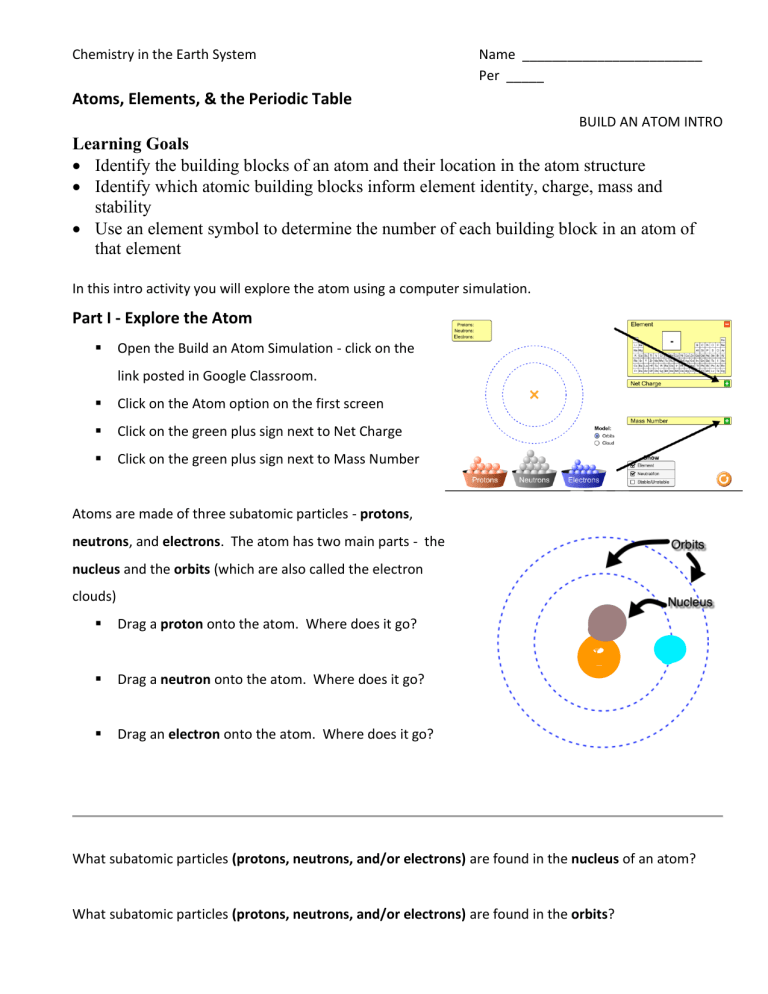
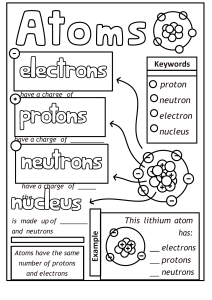
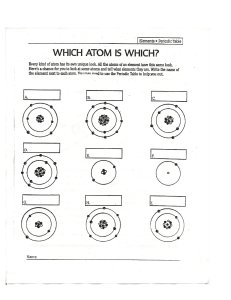
Chemistry in the Earth System Name ________________________ Per _____ Atoms, Elements, & the Periodic Table BUILD AN ATOM INTRO Learning Goals • Identify the building blocks of an atom and their location in the atom structure • Identify which atomic building blocks inform element identity, charge, mass and stability • Use an element symbol to determine the number of each building block in an atom of that element In this intro activity you will explore the atom using a computer simulation. Part I - Explore the Atom ▪ Open the Build an Atom Simulation - click on the link posted in Google Classroom. ▪ Click on the Atom option on the first screen ▪ Click on the green plus sign next to Net Charge ▪ Click on the green plus sign next to Mass Number Atoms are made of three subatomic particles - protons, neutrons, and electrons. The atom has two main parts - the nucleus and the orbits (which are also called the electron clouds) ▪ Drag a proton onto the atom. Where does it go? ▪ Drag a neutron onto the atom. Where does it go? ▪ Drag an electron onto the atom. Where does it go? What subatomic particles (protons, neutrons, and/or electrons) are found in the nucleus of an atom? What subatomic particles (protons, neutrons, and/or electrons) are found in the orbits? What element did you make when you added one proton, one neutron, and one electron? Hydrogen. ▪ Add another proton to the atom that you made. What changes? ▪ Add another neutron to the atom that you made. What changes? ▪ Add another electron to the atom that you made. What changes? What subatomic particle (proton, neutron, or electron) is responsible for determining the identity of an atom? What subatomic particles (protons, neutrons, and/or electrons) are responsible for determining the mass number of an atom? What subatomic particles (protons, neutrons, and/or electrons) are responsible for determining the net charge of an atom? Part II - Building Atoms of Elements ▪ Build the following atoms of elements. When each one is complete, record the protons, neutrons, and electrons in it, determine the identity of the element, and use colored pencils or markers to draw it on the model given. Directions Subatomic Particles What Element Diagram Example ▪ Add 1 proton p=1 ▪ Add 1 neutron n=1 ▪ Add 1 electron e=1 Directions Hydrogen H Subatomic Particles ▪ Add 5 protons p= 5 ▪ Add 6 neutrons n= 6 ▪ Add 5 electrons e= 5 ▪ Add 3 protons p= 3 ▪ Add 4 neutrons n= 4 ▪ Add 3 electrons e= 3 ▪ Add 10 protons p = 10 ▪ Add 10 neutrons n = 10 ▪ Add 10 electrons e = 10 ▪ Add 9 protons p= 9 ▪ Add 10 neutrons n= 10 ▪ Add 9 electrons e= 9 What Element Boron lithum neon flourine Diagram ▪ Add 6 protons p= 6 ▪ Add 6 neutrons n= 6 ▪ Add 6 electrons e= 6 carbon Part III - Identifying Atoms of Elements ▪ Give the mass number and Identify the elements given their diagrams and subatomic particles. Diagram Subatomic Particles Mass Number What Element p=4 n=5 beryllium 9 e=4 p=7 n=7 nitrogen 14 e=7 p=8 n=8 e=8 16 oxygen


![[C3-Worksheet Reference] Atoms, Elements & Compounds](http://s3.studylib.net/store/data/025600544_1-ac81b719e1b567117977c0e1deb5ecd6-300x300.png)
