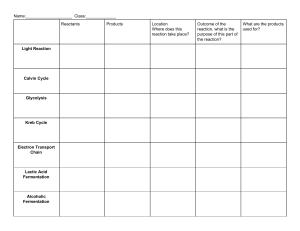
Organic Acids: Organic acid is a type of organic compound that typically has acidic properties. A common example of organic acids is called carboxyl acids, which are generally known as weak acids and do not totally dissociate in a medium such as water. Organic acids are widely distributed in nature as they occur in animal, plant, and microbial sources. They contain one or more carboxylic acid groups, which may be covalently linked in groups such as amides, esters, and peptides. Production of organic acids on a large industrial scale is mainly confined to acids of microbial origin. A number of organic acids of bacterial and fungal origin are important industrial products, the biological production of which has a definite economic advantage over chemical synthesis. A few examples of organic acids are: Lactic acid Citric acid Malic acid Acetic acid Formic acid Tartaric acid Lactic Acid: Lactic acid is an organic acid produced by the body when glucose (sugar) is broken down to generate ATP (cellular energy) in the absence of oxygen. It has a molecular formula CH3CH(OH)COOH. Lactic acid is produced industrially by bacterial fermentation of carbohydrates, or by chemical synthesis from acetaldehyde. Fermentative production Fermented milk products are obtained industrially by fermentation of milk or whey by Lactobacillus bacteria: Lactobacillus acidophilus, Lacticaseibacillus casei (Lactobacillus casei), Lactobacillus delbrueckii subsp. bulgaricus (Lactobacillus bulgaricus), Lactobacillus helveticus, Lactococcus lactis, Bacillus amyloliquofaciens, and Streptococcus salivarius subsp. thermophilus (Streptococcus thermophilus). As a starting material for industrial production of lactic acid, almost any carbohydrate source containing C5 and C6 sugars can be used. Pure sucrose, glucose from starch, raw sugar, and beet juice are frequently used. Lactic acid producing bacteria can be divided in two classes: homofermentative bacteria like Lactobacillus casei and Lactococcus lactis, producing two moles of lactate from one mole of glucose, and heterofermentative species producing one mole of lactate from one mole of glucose as well as carbon dioxide and acetic acid/ethanol. Chemical production Racemic lactic acid is synthesized industrially by reacting acetaldehyde with hydrogen cyanide and hydrolysing the resultant lactonitrile. When hydrolysis is performed by hydrochloric acid, ammonium chloride forms as a by-product; the Japanese company Musashino is one of the last big manufacturers of lactic acid by this route. Synthesis of both racemic and enantiopure lactic acids is also possible from other starting materials (vinyl acetate, glycerol, etc.) by application of catalytic procedures. Citric Acid: Citric Acid is a weak acid with a chemical formula C6H8O7. It can occur in two forms – monohydrate or water-free (anhydrous). This acid is usually found in citrus fruits like lemons, oranges etc. It is considered as a tribasic acid. It is odorless, sour in taste, and appears as a white crystalline solid. There are two production methods for Citric acid. 1. Biochemical method 2. Biological method Biochemical method: Citric acid is produced as a primary metabolite by the microorganisms. The microorganisms produce citric acid during trophophase. A high amount of sugar is transported through the pathway that forms acetyl CoA. The acetyl CoA condenses with oxaloacetic acid to yield “Citric acid” by the activity of an enzyme “Citrate synthetase”. Biological method: There are three methods for biological production of Citric acid. Koji process Surface culture process Submerged process Koji process: It is called “Solid-state fermentation. In Koji process, raw materials like apple, sugar cane, beet molasses are used. The Aspergillus niger utilizes raw materials. The raw materials pH and moisture content is adjusted to 4-5 and 70%. After that, inoculate A. niger. After inoculation, the medium is transferred into large trays of 3-5 cm depth and incubated at 2530 degrees Celsius for 7 days. At last, the citric acid is extracted from the fermentation tank. The raw materials starch content is degraded into citric acid by the amylase enzyme of the Aspergillus niger. Surface culture process: It is also called “liquid surface fermentation. In liquid surface fermentation, the culture medium (5-6 pH) is added to the aluminum shallow trays up to 5-20 cm deep. The process is carried out in the fermentation chamber. First, the spores of A.niger is blown onto the surface of the culture medium for about 5-6 days, and dry air is then passed. After 24 hours, the spores start to germinate, and the growth of white mycelium can be seen. In the surface culture process, a small amount of citric acid is produced as a primary metabolite by the A. niger. Submerged culture process: About 80% of the citric acid production is carried out through this method. Submerged fermentation makes the use of black Aspergillus, i.e., A. japonicus. It is performed in a bioreactor made of stainless steel compiled with proper aeration and cooling system. Malic Acid: Malic acid is an alpha hydroxy acid found in certain fruits and wines. It's used in foods and cosmetics, and sometimes as medicine. Malic acid is organic compound with the molecular formula C4H6O5. Malic acid is sour and acidic. This helps to clear away dead skin cells when applied to the skin. Its sourness also helps to make more saliva in people with dry mouth. Malic acid is also involved in the Krebs cycle. This is a process the body uses to make energy. Malic acid can be prepared by following methods: By partial reduction of tartaric acid: Malic acid can be prepared by the reduction of tartaric acid with HI CH(OH).COOH + 2HI CH(OH).COOH CH(OH).COOH+I2+H2O CH2-COOH From bromosuccinic acid: When bromosuccinic acid is treated with moist silver oxide malic acid is produced CHBr-COOH + Ag(OH) CH2.COOH CH(OH).COOH + AgBr CH2 . COOH Acetic Acid: Acetic acid (CH3COOH), also called ethanoic acid, the most important of the carboxylic acids. A dilute (approximately 5 percent by volume) solution of acetic acid produced by fermentation and oxidation of natural carbohydrates is called vinegar; a salt, ester, or acylal of acetic acid is called acetate. Industrially, acetic acid is used in the preparation of metal acetates, used in some printing processes; vinyl acetate, employed in the production of plastics; cellulose acetate, used in making photographic films and textiles; and volatile organic esters (such as ethyl and butyl acetates), widely used as solvents for resins, paints, and lacquers. Biologically, acetic acid is an important metabolic intermediate, and it occurs naturally in body fluids and in plant juices. Acetic acid is produced by following methods: 1. Synthetic method 2. Biological method Synthetic method A synthetic method for acetic acid production involves catalytic oxidation of acetaldehyde. To obtain acetic acid by the synthetic method, acetylene goes through catalytic hydration to form acetaldehyde, and later acetaldehyde undergoes catalytic oxidation to give acetic acid. The acetic acid formed by the synthetic method is called “Synthetic vinegar”. The synthetic vinegar is then subjected to the processes like dilution, aromatization and colouration. Dilution: The synthetic vinegar is first diluted via the addition of water. Aromatization: The aromatization of synthetic vinegar involves the addition of sugar, salts and a small amount of natural vinegar. Colouration: At last, the vinegar is coloured via the addition of caramel. A synthetic vinegar must be diluted in a way, that it must contain 4% of acetic acid per 100ml of water. The concentration of acetic acid will decide the strength of vinegar. The strength of vinegar is measured in grams and termed as “Gram strength”. Biological Method The biological method is generally employed for the commercial production of acetic acid through microorganisms like yeasts and bacteria. There are two methods, namely surface and submerged fermentation process widely used for the large-scale production. The fermentation of acetic acid can be anaerobic or aerobic. The anaerobic fermentation is carried out using Clostridium sp. of bacteria (in the absence of oxygen). Aerobic fermentation is primarily carried out by the yeasts (Saccharomyces cerevisiae) and secondly by the bacteria (Acetobacter aceti). There are two methods widely used for large scale Production of Acetic Acid Surface Fermentation: It uses trickling generator for the acetic acid production. Construction of trickling generator: The outer surface of the tank is composed of wood with the carrying capacity of 60m3. The inner surface contains the birch of wood shavings. Below the column of wood shaving, there is a cooling system at the bottom, which controls the heat generation during the process. The collecting chambers collect the liquid and recirculate it to the top for the conversion of ethanol into acetic acid. The acetic acid is drawn off through the outlet. Process: First, the ethanol is transferred into the generator from the inlet pump at the top. The ethanol is trickled through the column of wood shavings. The bacteria are inoculated at the bottom, which converts the ethanol into acetic acid through their microbial activity. A solution is cooled and pumped back to the top and again passed through the birch of wood shavings. The process is repeated until 80-90% of the ethanol converts into acetic acid. The concentration of ethanol should not be less than 0.2%, as it can increase the death rate of the microorganisms producing acetic acid. Therefore, for the optimum growth of microorganisms and production of vinegar, the concentration of ethanol must be in between 0.2-5.0%. The significant advantage of surface fermentation is that it leaves little residue with a high yield of acetic acid. Construction of the submerged fermentor: The submerged fermenter is made up of “Stainless steel”. The effigas turbine, at the bottom, allows continuous agitation. A suction rotor of the fermentor provides aeration. The heat exchange plates control the temperature of the culture medium. There is a foam separator, which eliminates the foam formed in the culture vessel. Through the harvester pump, the acetic acid is taken out. Process: Firstly, the ethanol is inoculated with the bacterial strain Acetobacter acetigenum. The fermentation is carried out for 35 hours at 40 degrees Celsius. The bacteria then grow and ferment the ethanol to acetic acid through continuous agitation and aeration. Submerged fermentation gives 10times/m3 higher yield than the surface fermentation and it is a cost-effective method. It requires only 20% of the plant area for the installation of the machine and does not require labour skills because of the automated machines. Formic Acid: It is a carboxylic acid. Its chemical formula is HCOOH. It is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. Esters, salts and the anion derived from formic acid are called formates. Industrially, formic acid is produced from methanol. Production From Methanol: Methanol is made inside our bodies from normal metabolic processes. It also enters the body from fruits and vegetables and their juices. In addition, humans produce methanol as well as aspartic acid and phenylalanine from the breakdown of aspartame, an artificial sweetener. Methanol is toxic, but most of us don't encounter enough of the chemical to be harmed. Inside our bodies, methanol is converted to formaldehyde, which is classified as a probable human carcinogen (cancer causer). However, the formaldehyde is rapidly transformed into formic acid and doesn’t collect in the body. The acid then leaves the body in urine or is changed into carbon dioxide and water. CH3OH + CO HOOCH3 Hydrolysis of this produces formic acid: HCOOCH3 + H2O HCOOH + CH3OH Scientists say that the production of formic acid from methanol in humans only becomes a problem if there is a large amount of methanol in the body, as there would be in methanol poisoning. In this situation, enough formic acid could be made to create a condition called acidosis. Symptoms of acidosis may include vision problems, blindness, memory loss, confusion, seizures, coma, low blood pressure, and cardiac arrest. Tartaric Acid: Tartaric acid is a white, crystalline organic acid that occurs naturally in many fruits, most notably in grapes, but also in bananas, tamarinds, and citrus. Its salt, potassium bitartrate, commonly known as cream of tartar, develops naturally in the process of fermentation. Its chemical formula is C4H6O6. Production By-products obtained from wine manufacturers for the basis for the commercial production of tartaric acid. The sediments and other waste products that result from the fermentation of wine are heated with calcium hydroxide, a base. KH(C4H4O6) + Ca(OH)2 Ca (C4H4O6) This causes calcium tartrate to form a precipitate, which is then treated with sulfuric acid to produce a combination of calcium sulfate and tartaric acid. Ca (C4H6O6) + H2SO4 H2 (C4H4O6) + C After separation, the tartaric acid is then purified for commercial use.