Proteins & Nucleic Acids: Structure, Classification, DNA & RNA
advertisement

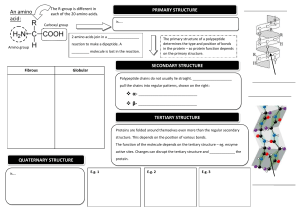
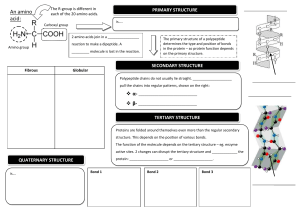
1.4 PROTEINS Learning outcomes: a) Describe the basic structure of amino acids. b) State how amino acids are grouped. c) Describe the primary, secondary, tertiary and quaternary levels of proteins and the types of bonds involved. d) Describe the effects of pH and temperature on the structure of proteins. e) Explain the formation and breakdown of dipeptide. f) Classify proteins according to structure and compositions. ❖ Biological macromolecules built from polymers of amino acids PROTEIN ❖ Main chemical constituents are carbon (C), hydrogen (H), oxygen (O) and nitrogen (N). ❖ Some also have sulphur (S) and phosphorus (P). The side chain / residual group R ✓varies in different amino acids. ✓determines the amino acid characteristics & proteins properties. AMINO ACID H2 N Amino group (NH2) C H COOH Carboxyl group AMINO ACID • Amino acids are amphoteric -consist both acidic carboxyl group & basic amino group • At cellular pH ( ~ 7.4 ) amino acids exist as bipolar ionic molecule i.e have positive and negative charge on same molecule ; known as zwitterions GROUPS OF AMINO ACID i. Non-polar side chain (hydrophobic) ii. Polar side chain (hydrophilic) iii. Acidic side chain (hydrophilic) iv. Basic side chain (hydrophilic) • Non-polar side chains ✓ ✓ ✓ usually the hydrocarbon. insoluble and unreactive (hydrophobic characteristics) usually forms structural proteins (such as collagen) • Polar side chains have electronegative atoms (such as oxygen and nitrogen) in their R groups produce partial charges (hydrophilic characteristics) (but do not receive or donate electrons) increases the solubility of proteins enables hydrogen bonding between polypeptide chains • Electrically charged side chains Acidic amino acids have second carboxyl group on the side chain (negatively charged) Basic amino acids have second amino group on the side chain (positively charged) • Amino acids are ✓ joined together by peptide bonds ✓ bonded together to form polypeptide chain • A protein is a biologically functional molecule made up of one or more polypeptides, each folded and coiled into a specific 3D structure LEVELS OF PROTEIN 1. Primary Structure •Specific linear sequence of amino acids in a polypeptide chain ; linked by peptide bond 2. Secondary Structure • Coiling/ folding of polypeptide chains due to formation of hydrogen bonds between the repeating constituents of the polypeptide backbone – helix TYPES – pleated sheet – helix • coiled polypeptide chain held together by hydrogen bond e.g. keratin found in hairs, nails, horn and feathers – pleated sheet • two or more segments of a polypeptide chain lie parallel to each other • hydrogen bonds hold the structure together e.g. silk protein 3. Tertiary Structure •Coiling / folding of the secondary structure to form a globular shape (3D shape) • resulting by interactions between R groups (side chains) of amino acids i) Hydrophobic & van der Waals interactions ii) Hydrogen bonds iii) Ionic bonds iv) Disulfide bridge i) Hydrophobic and van der Waals interactions • involving non polar side chains ii) Hydrogen bonds • between polar side chains iii) Ionic bonds • between positive and negative charged side chains iv)Disulfide bridge • between two cysteine amino acids ; amino acids with sulfhydryl groups ( -SH ) • Sulfur of one cysteine bonds to sulfur of other cysteine forming disulfide bridge e.g. myoglobin One polypeptide chain containing ironbearing heme group Function: oxygen storing pigment in muscle 4. Quaternary Structure •Combination of two or more polypeptide chains into one functional macromolecule e.g. Collagen, Haemoglobin Review: the four levels of protein structure EFFECT OF PH AND TEMPERATURE ON PROTEIN STRUCTURE Temperature ( extreme) • cause the breakage of : - hydrogen and ionic bonds - disulphide bridges - hydrophobic and van der Waals interactions • change in conformation of protein (denatured) pH ( extremely acidic or basic ) • cause the breakage of ionic bonds • change in conformation of protein (denatured) ❖ Most of denaturation are irreversible. Why? ✓ Denatured protein loses its threedimensional structure & functions. e.g. Albumin turns into insoluble egg white when proteins are denatured by high temperature. ❖ If the denatured protein remains dissolved, it may renature when the chemical and physical aspects of the environment are restored to normal How denaturation occurs at levels of protein structure Primary structure • not disrupted by denaturation. • Remain as sequence of amino acids held together by covalent peptide bonds Secondary structure • proteins lose all regular patterns (alpha-helixes & betapleated sheets) because of the disruption of hydrogen bonds. Tertiary structure Disruption of: ◦ Covalent bonds such as disulfide bridges ◦ Hydrogen bonds ◦ van der Waals & hydrophobic interactions ◦ Ionic bonds Quaternary structure •Dissociation of protein sub-units. •Disruption of the arrangement of the subunits. FORMATION AND BREAKDOWN OF DIPEPTIDE • Two amino acids are positioned so that the carboxyl group of one is adjacent to the amino group of the other • They are joined by condensation reaction, with the removal of a water molecule forming a dipeptide • The resulting covalent bond is called a peptide bond • Breakdown of the peptide bond can be done by hydrolysis reaction FORMATION AND BREAKDOWN OF DIPEPTIDE Condensation Amino end Hydrolysis Carboxyl end POLYPEPTIDE •Polypeptide is a polymer of many amino acids linked by peptide bonds •Repeated sequence of (-N-C-C-N-C-C-) is the polypeptide backbone •Polypeptide has a free amino group (amino end) at one end and a free carboxyl group (carboxyl end) at the other end CLASSIFICATION OF PROTEIN BASED ON STRUCTURE AND COMPOSITION • conjugated • fibrous • globular 1. Fibrous protein • Most have secondary structure • Long, coiled strand/ threads • Static molecule • Insoluble in water • For support/ structure e.g. Keratin, collagen 2. Globular protein • Most have tertiary and some quaternary structure • Compact/ spherical form • Dynamic/ non-static molecule • Generally water soluble • Biological agent/ catalyst/ metabolic function e.g. immunoglobulin ( antibody ), enzyme, hemoglobin, peptide hormone 3. Conjugated protein • Consist of amino acids & non-protein materials/ prosthetic group Conjugated Protein Prosthetic Group Location myoglobin haem (with iron) muscle haemoglobin haem (with iron) erythrocyte nucleoprotein nucleic acid chromosomes cytochrome oxidase copper electron carrier 1.5 DNA and RNA molecules Learning outcomes: a) State the structures of nucleotide as the basic composition of nucleic acids b) Illustrate the structure of DNA based on the Watson and Crick Model c) State the types of RNA d) Compare DNA and RNA NUCLEIC ACID ▪ Nucleic acids are macromolecules that exist as polymers called polynucleotides ▪ Nucleic acids are composed of hydrogen, oxygen, carbon, phosphorus and nitrogen. ▪ Two types of nucleic acids: DNA and RNA TYPES OF NUCLEIC ACIDS DNA Deoxyribonucleic acid RNA Ribonucleic acid NUCLEOTIDES ▪ Each polynucleotide made up of basic units called nucleotides ✓ Individual nucleotide comprises of 3 parts : o Phosphate group o Pentose sugar o Organic base / Nitrogenous base NUCLEOTIDE A nucleotide Phosphate group • Derived from phosphoric acid • Give acidic property to nucleic acids Pentose sugar A nucleotide Nitrogenous base A nucleotide NUCLEOTIDE Phosphoester linkage Glycosidic linkage ❖ Phosphoester linkage links pentose sugar and phosphate group (sugar-phosphate backbone). ❖ The bond between nitrogenous base and pentose sugar is glycosidic linkage. DINUCLEOTIDE • Combination of two nucleotides through condensation. Phosphoester linkage POLYNUCLEOTIDE • Continued condensation forms polynucleotide. • Phosphate group 3rd is linked to the carbon of one pentose sugar & to the 5th carbon of the next pentose sugar • Nitrogenous base is linked to the 1st carbon of the pentose DNA • Consists of two polynucleotide strands (coiled together to form double helix) • Contains 4 bases: i. Adenine (A) ii. Guanine (G) iii. Cytosine(C) iv. Thymine (T) ✓ A always pair with T ✓ C always pair with G ✓ The amount between A&T and C&G is equal • 2 strands run in opposite directions i.e anti-parallel: 5’ → 3’ and 3’ → 5’ • Both strands are held together by hydrogen bond between complementary base pairs • A&T ( linked by 2 hydrogen bonds ) • C&G ( linked by 3 hydrogen bonds ) • Each full turn of the helix has 10 base pairs • Sugar & phosphate molecules are linked by phosphodiester bond to form sugar-phosphate backbones One complete turn : (3.4 nm long) contains 10 bases pairs Sugar-phosphate backbone RNA • Single stranded polymer of nucleotide. • Pentose sugar : ribose • Organic bases : Guanine, Cytosine, Adenine and Uracil (replacing thymine) • 3 types of RNA : i. Ribosomal RNA (rRNA) ii. Transfer RNA (tRNA) iii. Messenger RNA (mRNA) COMPARISON BETWEEN DNA AND RNA The similarities between DNA and RNA 1. Both are made of monomers called nucleotides 2. Both contain pentose sugars 3. Both have the three nitrogenous bases: Adenine, Cytosine & Guanine 4. Both have phosphate groups in their nucleotides 5. Both are necessary for the cell to synthesise proteins. The differences between DNA and RNA DNA • Consists of two polynucleotide strands • Forms double helix • Larger molecular mass • Deoxyribose as pentose sugar • Nitrogenous bases: A,T,C,G RNA • Consists of one polynucleotide strands • No double helix is formed • Smaller molecular mass • Ribose as pentose sugar • Nitrogenous bases: A,U,C,G The differences between DNA and RNA • • • • • DNA Manufactured in nucleus Chemically very stable Permanent Only one basic form Ratio of A and T to C and G is one • • • • • RNA Manufactured in nucleus but found throughout the cell Chemically unstable Temporary existing 3 basic forms Ratio of A and U to C and G varies