
Drug Discovery Today d Volume 27, Number 11 d November 2022 REVIEWS María González-Rodríguez a,b,1, Clara Ruiz-Fernández a,c,1, Alfonso Cordero-Barreal a,d, Djedjiga Ait Eldjoudi a, Jesus Pino a,e,2,⇑, Yousof Farrag a,2,⇑, Oreste Gualillo a,2,⇑ a SERGAS (Servizo Galego de Saude) and NEIRID Lab (Neuroendocrine Interactions in Rheumatology and Inflammatory Diseases), Research Laboratory 9, IDIS (Instituto de Investigación Sanitaria de Santiago), Santiago University Clinical Hospital, Santiago de Compostela, Spain b International PhD School of the University of Santiago de Compostela (EDIUS), Doctoral Programme in Drug Research and Development, Santiago de Compostela, Spain c International PhD School of the University of Santiago de Compostela (EDIUS), Doctoral Programme in Medicine Clinical Research, Santiago de Compostela, Spain d International PhD School of the University of Santiago de Compostela (EDIUS), Doctoral Programme in Molecular Medicine, Santiago de Compostela, Spain e Departamento de Cirurgía y Especialidades Médico-Cirúrgicas àrea de Traumatología e Ortopedia, Universidade de Santiago de Compostela, Santiago de Compostela, Spain Abbreviations: ADAMTS, A disintegrin and metalloproteinase with thrombospondin motifs; AF, Annulus fibrosus; AMPK, AMP-activated protein kinase; AS, Ankylosing Spondylitis; APJ, Angiotensin domain type 1 receptor-associated proteins.; BMC, Body Mineral Content; BMD, Bone mineral density; BMMs, Bone marrow-derived monocytes; BM-MSCs, Bone marrow-mesenchimal stem cells; CAMKII, CaM kinase II; COX-2, Cyclooxygenase-2; CRP, C-reactive protein; DAS28, 28-joint count Disease Activity Score; eNAMPT, Extracellular nicotinamide phosphoribosyltransferase; ERK, Extracellular-signalregulated kinase; ESR, Erythrocyte sedimentation rate; ICAM-1, Intercellular Adhesion Molecule 1; ICTP, Carboxyl-terminal telopeptide of type I collagen; IFN-c, Interferon gamma; IPFP, Infrapatellar fat pad; IGF-1, Insulin-like growth factor; IKKab, Inhibitor of nuclear factor kappa-B kinase subunit alpha beta; IL, Interleukin; iNAMPT, Intracellular nicotinamide phosphoribosyltransferase; IVDD, Intervertebral disc degeneration; JNK, cJun N-terminal kinases; LCN-2, Lipocalin-2; LKB1, Liver kinase B1; MCP-1, Monocyte chemoattractant protein 1; MetS, Metabolic syndrome; MMP, Matrix Metallopeptidase; mPGES-1, Microsomal prostaglandin E synthase-1; MSC, Messenchimal stem cells; NAMPT, Pre-B cell colony-enhancing factor of nicotinamide phosphoribosyltransferase; NF-jB, Nuclear factor- jB; NO, Nitric oxide; OA, Osteoarthritis; P1NP, Procollagen 1 amino-terminal propeptide; p38-MAPK, p38 mitogen-activated protein kinases; PBMCs, peripheral blood mononuclear cells; PGE2, Prostaglandin E2; PI3K, phosphoinositide 3-kinase; PKB, Protein kinase B; PPAR, peroxisome proliferator-activated receptor; RA, Rheumatoid arthritis; RANKL, Receptor Activator for Nuclear Factor-jB Ligand; RASFs, RA synovial fibroblasts; ROS, Reactive oxygen species; SLE, Systemic lupus erythematosus; STAT, Signal transducer and activator of transcription; TGF-b, Transforming growth factor beta; TIMP-2, Tissue inhibitor of metalloproteinase 2; TLR4, Toll-like receptor 4-dependent; TNF-a, Tumor necrosis factor a; VCAM-1, Vascular cell adhesion molecule 1; WAT, White adipose tissue. KEYNOTE Adipokines as targets in musculoskeletal immune and inflammatory diseases Dr. Oreste Gualillo graduated in pharmaceutical chemistry and technology from the School of Pharmacy, University ‘Federico II’, Naples, Italy. He was awarded a PhD in pharmacology in 1996. He received postdoctoral training in molecular endocrinology at the Necker School of Medicine, INSERM U344, in Paris. From 1998 to 2000, he was a Marie Curie TMR30 post-doctoral researcher at the Department of Medicine, Molecular Endocrinology Division of the University of Santiago de Compostela. In 2001, he set up his own research group at the Santiago University Clinical Hospital, Division of Rheumatology. He is currently a staff researcher in SERGAS in the Spanish National Health System, at Area Sanitaria de Santiago de Compostela e Barbanza, Santiago University Clinical Hospital, heading the Neuroendocrine Interactions in Rheumatology and Inflammatory Diseases (NEIRID) group of IDIS (Instituto de Investigación Sanitaria de Santiago de Compostela). He works on the regulation and signal transduction of adipokines in inflammatory, rheumatic, and musculoskeletal diseases. Dr. Yousof Farrag graduated in pharmaceutical sciences from the University of Alexandria, Egypt. He was awarded a PhD in applied physics in 2018 from the University of Coruña, Spain. He received postdoctoral training in the Institute of Polymer Physics of the Spanish National Research Council (CSIC). He is currently a postdoctoral researcher of the Sara Borrell program of the Spanish National Institute of Health (Instituto Nacional de Salud Carlos III) leading a project on the role of specific biomaterials and hydrogels for drug delivery and regenerative medicine, with a particular emphasis to cartilage applications. He is also a staff researcher in the NEIRID group, IDIS. ⇑ Corresponding authors.Pino, J. (jesus.pino@usc.es), Farrag, Y. (yousof.farrag.zakaria@sergas.es), Gualillo, O. (oreste.gualillo@sergas.es). 1 2 These authors contributed equally to this work. These authors contributed equally to this paper and share senior co-authorship. 1359-6446/Ó 2022 Elsevier Ltd. All rights reserved. https://doi.org/10.1016/j.drudis.2022.103352 www.drugdiscoverytoday.com 1 KEYNOTE Drug Discovery Today KEYNOTE Adipokines are the principal mediators in adipose signaling. Nevertheless, besides their role in energy storage, these molecules can be produced by other cells, such as immune cells or chondrocytes. Given their pleiotropic effects, research over the past few years has also focused on musculoskeletal diseases, showing that these adipokines might have relevant roles in worsening the disease or improving the treatment response. In this review, we summarize recent advances in our understanding of adipokines and their role in the most prevalent musculoskeletal immune and inflammatory disorders. d Volume 27, Number 11 d November 2022 Dr. Jesus Pino graduated in medicine in 1979 and obtained a specialty degree in orthopedic surgery in 1986. He was awarded a PhD in medicine in 2008 studying physical and biological properties of new biomaterials in orthopedic surgery. He is currently surgeon at the orthopedic surgery division of the Santiago University Clinical Hospital. He is also a professor of orthopedic surgery at the University of Santiago, Faculty of Medicine. He is also a staff researcher in the NEIRID group, IDIS. Keywords: Adipokines; Inflammation; Arthritis; Musculoskeletal diseases; Metabolic Syndrome Introduction Adipose tissue is no longer considered just an energy reservoir: it also has a relevant role as a complex multifunctional endocrine organ releasing bioactive polypeptides called adipokines. Over the past few decades, adipokines were shown to have a role in inflammation, immunity, blood pressure, and lipidic and energetic metabolism. They are released principally by white adipose tissue (WAT), mainly via adipocytes, although also by preadipocytes, adipose tissue-infiltrated immune cells, and other cell types within adipose tissue. In addition, they can also be synthesized in other tissues, such as bone, cartilage, and immune cells.1 Over the past few decades, the relevance of adipokines in inflammatory mechanisms, and metabolic, cardiovascular, and musculoskeletal diseases (MSDs) has been assessed. Many adipokines, such as leptin, resistin, or adiponectin, are related not only to metabolic diseases because of their role regulating energetic homeostasis, but also to inflammatory diseases because they might also have pro- or anti-inflammatory effects. Musculoskeletal and immune-inflammatory diseases, such as osteoarthritis (OA), rheumatoid arthritis (RA), intervertebral disc degeneration, and osteoporosis, have an increasing impact in developed countries because of lifestyle factors, obesity incidence, and increased life expectancy. Over 344 million cases of OA and 14 million cases of RA were reported in 2019 worldwide.2 These chronic diseases are also associated with significant economic burdens. Most of these diseases cannot be cured; instead, current therapies only treat symptoms and entail complex surgical interventions that are associated with slow recuperation and time-consuming rehabilitation. These immune-inflammatory disorders have been described to be associated with high levels of certain adipokines in synovial fluid and blood. Recent research correlates adiponectin with bone destruction and RA risk, reports increased leptin and resistin levels in patients with OA, and reveals a role for chemerin in osteoporosis promoting osteogenic differentiation and bone formation. In this review, we summarize recent discoveries related to the impact of adipokines in MSDs, focusing on the principal adipokines, such as leptin, adiponectin, visfatin, resistin, or lipocalin 2 (LCN2), and new adipokines discovered more recently. 2 www.drugdiscoverytoday.com Adipokines Leptin Leptin is a 16-kDa nonglycosylated hormone encoded by the obese (ob) gene and synthesized mainly by WAT. Levels of circulating leptin are dependent on the WAT mass in addition to the nutritional status. Glucocorticoids, insulin, sex steroids, cytokines and toxins are reported to be involved in the regulation of leptin expression.3–6 Leptin receptors (Ob-R) are a class I cytokine receptor family encoded by the diabetes (db) gene. At least six Ob-R isoforms have been identified: four short isoforms (ObRa, Ob-Rc, Ob-Rd, and Ob-Rf), a soluble isoform (Ob-Re), and a long isoform (Ob-Rb), which has a full intracellular domain that affects signal transduction via the canonical Janus kinase (JAK) and signal transducer and activator of transcription (STAT) signaling pathways. They share the same extracellular binding domains but have different cytoplasmic domain lengths. In addition, leptin receptors act via extracellular signal-regulated kinases (ERKs) 1/2, c-Jun N-terminal kinases (JNKs), p38 mitogenactivated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K)/Akt pathways, protein kinase C (PKC), and Src homology region 2-containing protein tyrosine phosphatase 2 (SHP2)/growth factor receptor-bound protein 2 (GRB2). Leptin receptors are expressed throughout the innate and adaptive immune system cells, blood vessels, and cardiomyocytes.7 Previous studies showed elevated leptin levels in the serum and synovial fluid of patients with RA compared with healthy controls.8 Another study reported a significant correlation between serum leptin and reduced joint damage, RA duration, and the levels of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), 28-joint count Disease Activity Score (DAS28), tumor necrosis factor a (TNFa), and interleukin 6 (IL6).9 Nevertheless, no significant differences in plasma and synovial fluid leptin levels were found between patients with RA and healthy individuals; neither were correlations to disease duration, disease activity, CRP, ESR, radiographic factor, erosive or non-erosive RA.10 Leptin was reported to promote RA fibroblast-like synoviocyte (FLS) migration and angiogenesis via increasing the production of reactive oxygen species (ROS). Antagonists of IL6, IL1, and TNF reduced the leptin-induced ROS production and FLS migration.11 RA has been associated with regulatory CD4+Foxp3+ d Volume 27, Number 11 d KEYNOTE November 2022 KEYNOTE T cells (Treg) defects. Leptin receptors are expressed on the surface of Treg cells. Leptin monoclonal antibodies neutralize leptin and cause reversion of the hyper-responsiveness to anti-CD3 and anti-CD28 stimulation and the promotion of Treg expansion. This proliferation can be reversed using recombinant leptin. These results suggest that leptin acts as a negative signal for the expansion of Foxp3+CD25+CD4+ and might be a potential therapeutic approach for autoimmune diseases.12 Several studies showed a correlation between leptin levels in serum or synovial fluid and OA. Leptin levels in patients with knee OA were higher than in controls, with even higher expression in female than in male patients.13 Another study also reported a correlation between leptin levels and WOMAC-pain score and the radiographic stage of knee OA, enhancing OA progression.14 The leptin receptor Ob-Rb is highly expressed in OA cartilage.15 Moreover, leptin was reported to activate the mTOR pathway in chondrocytes, leading to the inhibition of autophagy, which, in turn, was reversed by the blockade of mTOR.16 Higher amounts of leptin secretions in synovium and infrapatellar fat pad (IPFP) were reported in patients with OA with metabolic syndrome (MetS).17 Leptin mediates the association of OA with obesity, a potent risk factor for OA incidence and progression.18 Accordingly, significantly higher leptin levels were reported only in patients with knee OA with body mass index (BMI) > 30.14 In a rat model of osteoporosis, Zheng et al. showed that leptin overexpression in bone marrow stromal cells enhanced periodontal regeneration.19 Another study showed that miR29 decreased leptin expression in adipocyte development and reduced high fat diet-induced leptin expression in bone, controlling leptin signaling to maintain bone anabolism.20 Segar et al. showed that leptin can interfere with intervertebral disc cell metabolism, increasing protease and nitric oxide (NO) production. It also synergized with pro/inflammatory mediators enhancing metalloproteinase, NO, and cytokine production21 Another study showed that in mice with type 2 diabetes mellitus induced by leptin receptor knockout, intervertebral disc degeneration (IVDD) was triggered by higher levels of matrix metallopeptidase (MMP)-3 and cell apoptosis promotion.22 Regarding leptin signaling in IVDD, a study showed that, in leptin receptorknockout mice, only female animals presented altered disc morphology, indicating delayed disc cell proliferation and differentiation, instead of degeneration.23 Recent studies reported serum leptin levels to be higher in patients with systemic lupus erythematosus (SLE), although there was no observed correlation with disease activity.24–25 As in RA, leptin systemic levels were associated with a deficiency of Treg cells.26 B cells stimulated with either leptin or serum of patients with SLE produced increased levels of IL6, IL10, and TNFa.27 Anti-leptin antibodies were able to reverse the Treg deficiency and cytokine overproduction. A recent study in mice indicated that leptin deficiency can protect against SLE, although, in a spontaneous SLE mouse model, leptin can accelerate the pathology.28 Several studies did not find a correlation between serum leptin levels and ankylosing spondylitis (AS) development.29–30 Instead, leptin was associated with bone protection and new bone formation in AS.31 This might explain why women, who normally have higher systemic leptin levels compared with men, have less spinal structural damage than men in AS.32 Adiponectin Adiponectin is the most abundant adipokine in human blood.33 It is synthesized mainly by WAT and can be found in circulation in different isoforms, being the high-molecular-weight isoform the most important for glucose homeostasis.34 It has a positive role in metabolism, where its levels are negatively correlated with obesity, weight, and diabetes.35 Adiponectin also increases insulin sensibility in liver and muscle.36 In muscle cells, adiponectin triggers the oxidation of fatty acids through peroxisome proliferator-activated receptor a (PPARa) and AMP-activated protein kinase (AMPK).37–38 These circulation levels of adiponectin are lower in obese patients and can be regulated with drugs such as thiazolidinediones.36 Adiponectin has two main classical receptor subtypes, AdipoR1 and AdipoR2, on chromosomes 1, locus 1p36.13-q41, and 12, locus 12p13.31, respectively.39 As targets of the globular and full-length adiponectin, both receptors are associated with the activation of PPARa, PPARc, AMPK, glucose uptake, and fatty acid oxidation.36,40 AdipoR1 is related to the stimulation of AMPK, p38-MAPK, JNK, PPARa, and nuclear factor-kB (NF-jB) pathways, which inhibit gluconeogenesis and stimulate fatty acids oxidation. AdipoR2 is related to the action of PPAR pathways dissipating energy, stimulating fatty acids oxidation, and decreasing inflammation and oxidative stress.41 Adiponectin was also described to inhibit the inflammatory response, suppressing Toll-like receptor (TLR)-4-mediated NF-jB activation, polarizing M1 macrophages to M2, and T helper (Th)-1/Th17 to Th2/Treg.42 Multiple researchers have demonstrated the importance of adiponectin in the pathogenesis of synovitis in patients with RA. For instance, the group of Kusunoki found that adiponectin induces cyclooxygenase-2 (COX-2) and microsomal prostaglandin E synthase-1 (mPGES-1) expression, with increases in PGE2 production by RA synovial fibroblasts (RASFs).43 Adiponectin also enhances the production of IL6 and MMP1 through AMPK, p38, IKKab, and NF-jB in human synovial fibroblasts.44–45 Qian et al. suggested that adiponectin levels from synovial tissue in patients with RA can induce the production of osteopontin, which causes bone erosion.46 Some researchers also hypothesized that adiponectin could be useful as clinical biomarker in osteoporosis because of its role in bone metabolism.47–48 Compared with healthy controls, adiponectin showed higher levels in plasma and synovial fluid in patients with RA,46,49 whereas other studies showed no correlation with the clinical outcome of the disease.50–51 In addition, adiponectin levels were not even correlated to DAS28 or CRP levels.52 Most of the studies in cohorts of patients with OA showed that adiponectin levels are higher in these patients compared with controls; for instance, Wu et al. showed that adiponectin inhibits bone marrow-derived monocyte (BMM) growth and proliferation in a dose-dependent manner.53 Recently, the group of Orellana showed that the level of adiponectin in synovium was positively correlated with clinical severity in women with knee OA, and also with the production of IL6.54 www.drugdiscoverytoday.com 3 KEYNOTE Drug Discovery Today KEYNOTE The levels of adiponectin in IPFP were reported to be higher compared with subcutaneous adipose tissue in patients with OA.55 The particular profile of cytokines from adjacent cartilage in these patients could contribute to paracrine inflammation through the secretion of IL6/sIL6R.55 Adiponectin receptors are present in human chondrocytes,56 being reported to improve chondrogenesis and to be downregulated in patients with OA.57–58 In models of in vitro chondrogenesis (e.g., ATDC5 cells, derived from mouse terato-carcinoma),59 this adipokine increased type II collagen, Runx2, proliferation, synthesis of proteoglycans, and matrix mineralization.60 Plasma adiponectin levels were also reported to be higher in patients with OA than in healthy controls.61 These higher levels are correlated with the upregulation of MMP13 and PGE2 expression, because adiponectin increases the activity and production of PGE2,62 as well as proinflammatory mediators, such as NO, IL6, and other metalloproteinases,44,63 which indicates that this adipokine contributes to the pathology of OA. By contrast, adiponectin is being studied as an anti-inflammatory adipokine with anabolic functions increasing TIMP2 and decreasing MMP13 via IL1b.64 Adiponectin is able to increase adhesion molecules [e.g., intracellular adhesion molecule-1 (ICAM-1)] in human OA synovial fibroblasts.65 These adhesion molecules are important for the progression of OA because they mediate fibroblast adhesion and infiltration during OA pathogenesis. These molecules are activated via liver kinase B1 (LKB1)/CaM kinase II (CAMKII), AMPK, and the AP1 pathway, resulting in monocyte adhesion to human OA synovial fibroblasts.65 Bone-forming cells were reported to express adiponectin and its receptors,66 indicating the link between this adipokine and bone metabolism. Adiponectin levels were negatively correlated with bone mineral density (BMD) and body mineral content (BMC) levels.67 In addition, patients with MetS showed lower adiponectin levels compared with controls, which supports the negative correlation of adiponectin with BMD.68 Adiponectin is described to have opposite effects in bone: on the one hand, it stimulates the proliferation and mineralization of human osteoblasts via the p38 MAPK signaling pathway. On the other hand, it activates osteoclasts through RANKL and inhibits osteoprotegerin.69 In agreement with this, in the RAW 264.7 murine macrophage cell line, adiponectin was described to inhibit osteoclastogenesis and bone resorption, inhibiting the NF-jB and p38 signaling pathways, which are vital for osteoclast production.70 The group of Wang showed that adiponectin enhances osteogenesis via the Wnt/b-catenin pathway in bone marrow mesenchymal stem cells (BMSCs).71 New studies suggest that the role of adiponectin in bone metabolism depends on sex hormone levels because adiponectin levels are negative correlated with BMD in women, with no correlation in men.72 In human osteoblastic cells, adiponectin resulted in the upregulation of oncostatin M expression via the PI3K, Akt, and NF-jB pathways,73 which suggests it as a target for RA treatment. Krumblholz and colleagues examined adiponectin effects in bone, indicating that adiponectin upregulates the expression of IL8 and MMP9 in osteoclasts, and inhibits osteoprotegerin mRNA in osteoblasts, showing that adiponectin reduces mineralization and increases resorption in bone.74 4 www.drugdiscoverytoday.com Drug Discovery Today d Volume 27, Number 11 d November 2022 In AS, adiponectin levels are not correlated with the disease, although these levels are sex dependent and might be affected by androgens.75 Adiponectin levels were also not correlated with acute-phase reactant levels (e.g., CRP or ESR). Many studies reported that serum levels of adiponectin in patients with AS are not significantly different.30,32 Nevertheless, one study showed that adiponectin levels might be related to radiographic spinal cord evolution in patients with AS.32 Further studies are needed to clarify these relationships. Adiponectin levels in patients with SLE show no increase compared with healthy controls, which indicates no correlation with the disease.76–79 Visfatin Visfatin is a cytokine that was first isolated and characterized from a human peripheral blood lymphocyte cDNA library.80 It is also known as pre-B cell colony-enhancing factor of nicotinamide phosphoribosyltransferase (NAMPT), and is produced mainly by visceral adipose tissue, although its expression was also reported in adipose tissues, liver, muscle, and immune cells.81 Visfatin is correlated with obesity, with higher levels in obese subjects.82 It was also reported to be a useful protein for diagnostics, prognostics, and therapeutics in cardiovascular diseases.83 Visfatin inhibits insulin signaling, and the STAT3 and NF-jB pathways in the human liver cancer cell line HepG2;84 however, in osteoblasts, visfatin realized its insulin-like function via phosphorylation of the insulin receptor.85 Although there is no visfatin receptor described thus far, it was reported to bind to, and activate, the insulin receptor, mimicking the effect of insulin.81 Visfatin is involved in various signaling pathways and can be found in both intracellular (iNAMPT) or extracellular forms (eNAMPT). The extracellular form has a role in signaling pathways and is involved in some intracellular signaling cascades.83 The proinflammatory effects of visfatin in chondrocytes can be explained by activation of the insulin receptor signaling pathways.86 eNAMPT activates the ERK/MAPK pathway in chondrocytes, inhibiting insulin-like growth factor (IGF1) independent of the IGF1 receptor.87 The role of visfatin in bone metabolism has been studied in different populations, but remains controversial.88–89 For example, there was no significant correlation between visfatin and BMD in women with postmenopausal osteoporosis.90 Patients with MetS have higher levels of visfatin compared with controls, being positively associated with BMD, but only in men.68 Recent studies showed that visfatin does not affect BMD in women with primary osteoporosis.90 Terzoudis et al. described for the first time that visfatin levels and the presence of osteoporosis are positively associated in inflammatory bowel disease.91 Moreover, visfatin has been identified as a positive predictor of BMD in young survivors of acute lymphocytic leukemia.89 The suppression of visfatin was recently discovered to be accompanied by the suppression of osteoblast differentiation of bone marrow mesenchymal stem cells (BM-MSCs), indicating visfatin as a possible marker of osteoblast differentiation.92 At present, visfatin involvement in bone metabolism is not clear because of discrepancies between studies. KEYNOTE KEYNOTE d Volume 27, Number 11 d KEYNOTE November 2022 KEYNOTE Tsiklauri et al. found that visfatin is involved in matrix production in MSC differentiation and reduction of collagen type I expression, and can be an important player in the pathophysiology and development of osteoporosis, producing bone fragility.93 In addition, this research suggests that bone remodeling can be affected by visfatin producing proinflammatory factors, resulting in an MMP/TIMP imbalance.93 Visfatin has been reported to promote osteogenesis through the upregulating of Runx2 transcription.94 By contrast, visfatin also can increase its expression during inflammation.95 The suppression of visfatin in mouse BM-MSCs downregulates osteoblastogenesis, inhibits some osteoblast differentiation markers, alkaline phosphatase activity, and matrix mineralization of these cells.92 Recent studies of the mechanism of this adipokine demonstrated that visfatin suppresses RANKL-induced osteoclast differentiation by interfering with signaling pathways related to survival, such as JNK or Akt.96 Patients with RA or OA show an increase in the levels of visfatin expression in the synovium, being higher in RA.97 Visfatin was also demonstrated to increase the expression of several proinflammatory cytokines, such as IL1b, IL6, IL17A,TNFa, MMP1, MMP13, and antioxidant enzymes (SOD2, CAT, and NRF2).98–99 In particular, visfatin was reported to upregulate IL6 and TNFa expression, suppressing the expression of miR199a-5p via the ERK, p38, and JNK pathways in human synovial fibroblasts.100 Recently, visfatin was found to upregulate ICAM1 and stimulate monocyte adhesion to OA synovial fibroblasts by inhibiting the synthesis of miR320a via the AMPK and p38 pathways.101 These findings indicate a potential proinflammatory role of visfatin in synovium. The presence of visfatin and its relation with cartilage catabolism and inflammation were reported in patients with OA.102–103 Recent studies have discovered that visfatin is principally located in OA synovium in the human OA joint in its enzymatically active conformation.103 In chondrocytes, visfatin was shown to stimulate the secretion of IL6, Kc, and monocyte chemoattractant protein 1 (MCP1),103 which are important factors for the progression of OA. Results from meta-analysis studies showed higher visfatin levels in patients with RA than healthy controls, and even higher levels in patients with joint erosion.52,104–106 Visfatin, DAS28, and CRP levels were found to be positively correlated.52 It appears that overexpression of this adipokine inhibits osteoclast formation via Nfatc1.107–108 For instance, in a collagen-induced arthritis murine model, visfatin-deficient mice showed less bone destruction, inflammation, and RA progression.107 In conclusion, visfatin could be used as an inflammatory biomarker for RA, although other cytokines, such as chemerin, are more specific.109 Injecting visfatin to rat models aggravated the status of IVDD, as reported by the group of Cui.110 The same group showed that human nucleus pulposus cells treated with visfatin decreased collagen II and aggrecan levels, whereas MMP13 and IL6 increased.110 More studies are needed to confirm these findings. The levels of visfatin in patients with AS were higher than in healthy controls. This adipokine, as seen with adiponectin, is not correlated with acute-phase reactant levels (e.g., CRP or ESR).75 A clinical study in patients with AS without diabetes under TNFa antagonist therapy (infliximab) did not show any relation between visfatin and AS activity and systemic inflammation.111 Levels of visfatin and the progression of SLE do not appear to be correlated.78 By contrast, previous studies found that the levels of visfatin in patients with SLE are higher than in controls but not related to inflammation.112 Resistin Human resistin is a 12.5-kDa cysteine-rich adipokine that belongs to the family of ‘resistin-like molecules’ and is encoded by RETN. Two quaternary forms of resistin are present in humans: an abundant high-molecular-weight hexamer and a more bioactive trimer that induces hepatic insulin resistance as well as inflammation. Resistin is mainly expressed by monocytes and macrophages activated with lipopolysaccharide (LPS), IL1b, IL6, TNFa, and resistin itself. It is also expressed to a lesser extent by pancreatic b cell, lung cells, and placental tissue. Resistin enhances the expression of vascular cell adhesion molecule 1 (VCAM1), ICAM1, pentraxin 3, MCP1, TNFa, IL6, and IL12 through a NF-jB-dependent pathway in vascular endothelial cells, promoting leukocyte adhesion and the inflammatory response. Resistin competes with LPS for binding to TLR4 and adenylyl cyclase-associated protein 1 (CAP1). Resistin can signal through different receptors depending on the tissue and cell type [an isoform of decorin involved in WAT expansion, tyrosine kinase-like orphan receptor 1 (ROR1) in 3 T3-L1 cells, or IGF1 receptor (IGF1R) in fibroblasts]. Resistin activates G-proteindependent signaling, PI3K/Akt pathway, adenylate cyclase/ cAMP/PKA pathway, PKC, and extracellular Ca21 signaling through L-type voltage-sensitive Ca2+.41 Resistin can increase chemokine production by fibroblast-like synoviocytes in synovial tissue through the CAP1receptor.113 Li et al. reported that, in a Chinese population, resistin gene polymorphisms could determine genetic predisposition to RA.114 Another study provided further insights showing that high serum resistin levels were correlated with active inflammatory disease and faster radiological progression. They showed that TNFa production was increased by resistin in macrophages.115 A recent study did not find any association of resistin plasma levels with plasma chemokines, markers of inflammation, or disease activity in patients with newly diagnosed but untreated RA.116 Alissa et al. demonstrated that serum resistin levels were higher in patients with primary knee OA than in healthy controls. Resistin levels were also correlated with inflammatory markers and adiposity.117 A recent study showed that, in OA synovial fibroblasts, resistin upregulates VCAM-1 and enhances monocyte adhesion. Its signaling occurs via the p38, PKCa, and JNK pathways.118 Chen et al. described how resistin expression is higher in OA knee synovial tissue than in controls. This adipokine also triggers IL1b and TNFa expression in OA synovial fibroblasts through the ERK and MEK signaling pathways.119 In patients with OA, resistin was significatively increased only in those with high BMI.14 A recent study on patients with knee OA associated resistin-related metabolic inflammation with some knee structural changes, and confirmed the lack of evidence linking it with OA symptoms.120 A recent study also found that serum resistin levels are higher in postmenopausal women with low BMD and with osteopenia.121 Resistin was proved to increase ADAMTS5 expression in rat nucleus pulposus (NP) cells in a dose and time-dependent way, www.drugdiscoverytoday.com 5 KEYNOTE Drug Discovery Today KEYNOTE and that the p38 MAPK pathway is involved in the signaling.122 Another study confirmed these results in human NP cells, showing that, by triggering the p38 MAPK and NF-jB pathways, resistin, which binds TLR4, can upregulate CCL4 expression, triggering macrophage infiltration.123 A study assessing this issue did not show a significant association between resistin serum levels and SLE disease activity.124 Nevertheless, there are some studies that present contradictory data. Chougule et al. found that serum resistin levels were higher in patients with SLE than in healthy controls and that, in the SLE group, increased resistin levels correlated with patients presenting renal involvement.78 Supporting this study, a meta-analysis review stated that serum levels of resistin positively correlated with SLE disease activity.125 Besides confirming the correlation between patients with renal SLE involvement and higher resistin levels, resistin increment was also related to proteinuria and high creatinine levels.126 Finally, elevated serum resistin levels have been associated with MetS in patients with SLE.127–128 Resistin levels were also found higher in serum from patients with AS than in healthy controls. These high levels were correlated with radiographic progression and AS development, suggesting that it has a role in the pathogenesis of new bone formation instead of inflammation.30–31 Lipocalin-2 LCN2 is a glycoprotein produced mainly by WAT, although it is also expressed in spleen, liver, chondrocytes, and immune cells. It was first identified in human neutrophils granules and mouse kidney cells and is encoded by a gene located at the chromosome locus 9q34.11. It circulates as a 25-kDa monomer, 46-kDa homodimer, or as a covalent complex with MMP9, blocking MMP9 autodegradation. There are two proposed receptors to LCN2: the megalin/glycoprotein GP330, an LDL receptor that also binds to the human LCN2, and the transporter protein SLC22A17 (24p3R), which binds to mouse LCN2.7 Recently, LCN2 levels in serum were reported to be significantly higher in patients with RA than in healthy individuals. Within the group of patients with RA, higher LCN2 levels were positively correlated with high modified Larsen score (a scoring system based on structural damage observed on radiography), which can indicate an association with joint damage. However, no significant differences were observed between LCN2 serum levels and disease activity. Serum LCN2 levels in patients with RA were not directly correlated with inflammatory activation.129 LCN2 has been also suggested to be involved in the antiinflammatory regulation of M1/M2 polarization and in the increases in Treg cell proliferation. Another factor contributing to RA pathophysiology is the increase in LCN2 expression in neutrophils, caused by granulocyte–macrophage colonystimulating factor.7 LCN2 levels in synovial fluid were higher in patients with RA than with OA. Despite these recent findings, the role of LCN2 in the development of the disease continues to be largely unknown. LCN2 can contribute to the degradation of the cartilage matrix in OA via maintaining MMP9 activity.130 There is also evidence that LCN2 expression is inhibited throughout osteoblast differentiation and induced in osteoblasts and chondrocytes exposed to an OA-related inflammatory environment.131 The 6 www.drugdiscoverytoday.com Drug Discovery Today d Volume 27, Number 11 d November 2022 transcription factor E74-like factor 3 and NF-jB modulate the expression of LCN2 in chondrocytes. Of note, glucocorticoids used in OA and RA treatment were reported to increase LCN2 expression via corticoid receptor and PI3K, ERK1/2 and JAK2 pathways in immortalized mouse chondrocytes.7 Despite the increase in LCN2 levels in synovial fluid of OA knees, Maurizi et al. did not observe differences in LCN2 serum levels between patients with OA and normal BMD and healthy subjects.132 Although LCN2 has been associated with OA pathogenesis, no study has addressed its effects on catabolic or anabolic genes during OA pathogenesis. An in vivo study concluded that, in OA cartilage, Lcn2 upregulation was not sufficient or necessary for cartilage destruction in mice.133 However, a recent study found a significant upregulation of LCN2 in menisci during OA, suggesting that early-stage OA is affected by LCN2 activation in the menisci but not in chondrocytes.134 Evidence showed neither a correlation between LCN2 serum levels and BMD in patients with OA nor changes in LCN2 serum levels between patients with OA and healthy subjects.132 However, another recent publication showed that, in the MDX mouse model of Duchenne muscular dystrophy, the deletion of Lcn2, either genetically or blocking its activity with a monoclonal antibody, prevented or reverted bone loss induced by this disease.135 Despite the lack of new evidence on this topic, a study showed that, as in OA cartilage, in IVVD LCN2 can form a complex with MMP9, preventing its degradation and promoting its catabolic role. Lcn2 expression and MMP9 activity were also upregulated by nerve growth factor in annulus fibrosus (AF) cells, promoting the degeneration of AF tissue in vivo.136 In a study comparing patients with SLE with healthy controls, no significant difference was found in serum LCN2 levels.137 However, a recent study proposed cerebrospinal fluid LCN2 as a reliable biomarker of neuropsychiatric SLE.138 In a mouse model, Lin et al. found that serum LCN2 levels were significantly higher in the ank/ank model than in the wild type used as control and those levels in the ankylosis model were positively correlated with disease score. In addition, circulating LCN2 levels were modulated by PPARc, which might be a potential pathway involved in inflammation and ankylosis in AS. More studies are needed to elucidate the mechanism of action of PPARc regulating Lcn2 expression.139 Chemerin Chemerin has been proposed as a link between RA disease activity and treatment response, mediating at the same time chronic inflammation and obesity. Plasma chemerin levels decreased after weight loss, improving RA state in overweight or obese patients.140 Vazquez-Villegas et al. reported higher serum chemerin levels in patients with RA with functional disability compared with patients with RA without a disability.104 In a recent study evaluating serum chemerin as a biomarker of RA disease activity, patients with RA with and without diabetes mellitus showed no differences in serum chemerin levels. A positive correlation was detected between serum chemerin levels and clinical variables such as DAS-28-CRP, serum CRP levels, and swollen joint count. Chemerin levels in patients with moderate or severe RA activity (DAS > 2.9) were higher than those with RA in remission/mild disease activity (DAS < 2.9). Patients with RA and high KEYNOTE KEYNOTE d Volume 27, Number 11 d KEYNOTE November 2022 KEYNOTE chemerin levels (103 ng/ml) were shown to have a higher risk of moderate or severe disease activity.141 Recently, it was shown that the serum chemerin levels of patients with RA increased after 6 months of TNFa inhibition, but decreased after 1 year of anti-TNF therapy.142 Chemerin levels observed in synovial fluid and membrane were higher in patients with OA than in patients with non-OA joint diseases. In addition, these levels were positively correlated with disease severity.143 Another study also confirmed this positive correlation, although no association was found between serum chemerin levels and OA severity.144 A cohort study reported that patients with hip, knee, or hand primary OA presented higher serum chemerin levels compared with healthy individuals, although they did not show significant associations with the radiologically diagnosed severity of the disease.145 In an in vitro study, chondrocyte cells stimulated with chemerin reduced the proliferative capacity, presenting a synergism with IL1b stimulation. Assessing gene expression, both treatments were confirmed to cause downregulation of MMP1, MMP3, MMP13, and all protective genes in OA. This study also found that phosphorylation of Akt/Erk pathway was involved in the signaling.146 Muruganandan et al. investigated the mechanism involved in MSC adipogenic and osteoblastogenic differentiation mediated by chemokine-like receptor 1 (CMKLR1) signaling. They demonstrated that chemerin/CMKLR1 modulates canonical Wnt signaling in MSCs and that CMKLR1 is a Wnt-responsive gene that causes a negative feedback loop to restrain osteoblastogenic Wnt signaling. They also proved Notch signaling involvement in this process.147 It has also been shown that the chemerin receptor is expressed in osteoclasts, and that its protein expression is higher in mature osteoclasts compared with preosteoclasts. In addition, the authors showed that chemerin enlarges the functional resorption capacity of osteoclasts and that ERK5 activation is involved in chemerin regulation of osteoclast activity.148 A study assessing the role of another chemerin receptor, GRP1, in bone metabolism showed that GPR1 deficiency caused severe osteoporosis associated with lower sex hormones in serum in male mice.149 Finally, Li et al. reported that chemerin in bone marrow promotes osteogenic differentiation and bone formation in vitro and in vivo through Akt/Gsk3b/b-catenin signaling activation.150 In a recent publication assessing this topic, Hu et al. showed that levels of the expression of chemerin and its receptor CMKLR1 in NP tissue were markedly increased with a higher degeneration grade. They also observed that the expression levels were lower in AF tissue. The authors reported a positive correlation of serum chemerin levels with lipid metabolism and higher chemerin levels in an obese group versus a control group. The same study showed that chemerin can reduce collagen II, aggrecan, and SOX9 expression, and promote the expression of MMP9, MMP13, and ADAMTS5, resulting in inhibition of ECM synthesis, enhancement of its degradation, and disruption of the ECM synthesis balance. They also found that NF-jB and upstream AKT phosphorylation and TLR4 and CMKLR1 receptors were involved in chemerin signaling in NP cells. Finally, Hu et al. showed that in vivo chemerin overexpression increased the loss of disc height, enhancing IVDD progression.151 Chougule et al. showed no significant differences in serum chemerin levels between patients with SLE and healthy controls.78 The role of chemerin in AS has not yet been elucidated. In a study assessing the link between adipokines and radiographic spinal progression in patients with AS, no differences were observed in serum chemerin levels during the spinal progression stages.32 Adipsin Adipsin is an adipogenesis marker gene in adipocyte differentiation of BM-MSCs and is secreted by adipocytes, promoting their differentiation.152 Adipsin serum levels did not show significant differences between patients with early stages of RA and healthy subjects.153 In patients with RA with comorbid type 2 diabetes mellitus, a significant reduction in adipsin serum levels was observed after 6 months of IL1-blocking agent administration, whereas no change was detected in patients treated with a TNF inhibitor.154 Adipsin is secreted from the IPFP, which has previously been implicated in OA pathogenesis. It has also been used as a MetS serum marker in patients with OA and is recognized to be implicated in joint tissue homeostasis.155–156 A study found that IPFP expression of adipsin is suppressed by moderate exercise, which appears to be mediated by CITED2, a mechanosensitive transcriptional regulator with chondroprotective activity that might have a role in the modulation of adipsin and other adipokines secreted from the IPFP.157 In patients with OA, serum and cartilage adipsin levels were higher compared with non-OA controls. A recent study evaluated a group of adipokines and inflammatory factors and their serum ratios in patients with OA classified according to their BMI. An increase in the adipsin/MCP1 ratio in obese subjects was only associated with lateral compartment knee cartilage volume loss over time. This might indicate that, in patients with a higher BMI, adipsin have a predominant role in lateral compartment cartilage loss above that reported for other potential factors.144 An exploratory study also found that leptin levels and systemic inflammation were positively correlated with higher adipsin levels.158 Further analysis confirmed that higher baseline values of adipsin in serum were associated with greater cartilage volume loss in the lateral compartment in an OA population at a mild to moderate stage of the disease.159 Adipsin levels were significantly higher in the serum and synovial fluid of patients with OA. Both synovial membranes and chondrocytes expressed high levels of adipsin, although its expression in synoviocytes, especially in osteoblasts, was low.159 Using a mouse model, Valverde-Franco et al. demonstrated the in vivo role of adipsin in OA as a contributing factor to knee tissue degradation. They observed that the alterations in cartilage and synovial membrane were significantly reduced in the absence of adipsin. They reported that adipsin might be involved in the pathophysiology of OA by activating the alternative complement pathway. This study showed that adipsin expression in human OA synovial membrane and cartilage is higher than controls. By contrast, adipsin expression levels in joint cells were similar in both OA and control.159 Recently, a study characterized adipsin serum levels from patients with knee OA as CRP-related metabolic inflammation and positively associated it with the symptoms, but not with knee structural changes.120 In a recently published study on postmenopausal women, no differences were observed in median circulating adipsin levels www.drugdiscoverytoday.com 7 KEYNOTE Drug Discovery Today KEYNOTE Drug Discovery Today d Volume 27, Number 11 d November 2022 KEYNOTE between the low BMD group and the control group.160 Another study reported significantly higher serum levels of adipsin in postmenopausal women with low BMD compared with women with normal BMD. They also found that adipsin circulatory levels were not affected by the patient age, but were affected by years since menopause.121 As with other adipokines, adipsin contributes to low-grade inflammatory response in autoimmune diseases such as SLE, although its pathophysiology is not yet clear. Chougule et al. reported significantly elevated serum adipsin levels in patients with nontreated SLE compared with healthy controls, although no correlation was found with disease activity. They also reported that adipsin serum levels were significantly higher in patients with SLE with renal involvement compared with those without renal involvement.78 To the date, no research has been published regarding the role of adipsin in IVDD and AS.(See Figs. 1-5). Apelin Apelin is a peptide that binds specifically to the angiotensin domain type 1 receptor-associated proteins (APJs), which belong to the G-protein-coupled receptor family.161 This apelin/APJ system is present around the body.162 The lack of apelin could suppress the Wnt/b-catenin pathway, which is the principal reason why apelin is correlated with osteoporosis. In addition, the lack of apelin decreases lysyl oxidase (LOXL)-3 and LOXL4 expression,163 which participate in collagen fiber production, and have an impact on osteoblast formation. Apelin is related to the procollagen 1 N-terminal propeptide (P1NP) and C-terminal telopeptide of type I collagen (ICTP). Recent studies showed that apelin is positively correlation with PINP, but negatively with ICTP.164 Drug Discovery Today FIGURE 2 Schematic of the principal actions of adiponectin in bone and synovia. Adiponectin leads to cartilage and synovia inflammation by increasing nitric oxide synthase 2 (NOS2), interleukin (IL)-6, IL8, and metalloproteinases. It also contributes to bone erosion, decreasing its mineralization and increasing its degradation. For additional defintions, please see the main text. Apelin has been identified as a predicting indicator for arthritis. Recent studies described how apelin stimulates IL1b expression Drug Discovery Today FIGURE 1 Schematic of the role of leptin in the musculoskeletal diseases. On the one hand, leptin can induce disc degradation by increasing protease and nitric oxide (NO) secretion, which contributes to intervertebral disc degeneration (IVDD). On the other hand, leptin reduces chondrocyte autophagy, induces chondrocyte senescence, increases reactive oxygen species (ROS) production and migration of immune cells, and leads to cartilage degradation and inflammation. For additional defintions, please see the main text. 8 www.drugdiscoverytoday.com d Volume 27, Number 11 d November 2022 KEYNOTE KEYNOTE KEYNOTE Drug Discovery Today Drug Discovery Today FIGURE 3 Schematic of the action of visfatin action in different tissues in osteoarthritis (OA) and rheumatoid arthritis (RA). Visfatin levels increase in degraded cartilage and increase with disease stage, stimulating the secretion of inflammatory signals. For additional defintions, please see the main text. Drug Discovery Today FIGURE 4 Schematic of the role of resistin in osteoarthritis (OA) and rheumatoid arthritis (RA). Resistin can induce synovial fibroblast vascular cell adhesion molecule 1 (VCAM1) upregulation and chemokine overexpression by fibroblast-like synoviocyte (FLS) and macrophage-like interleukin (IL)-1b and tumor necrosis factor (TNF)-a, promoting monocyte adhesion and inflammation. via PI3K and ERK.165 Both apelin and IL1b have roles in OA as proinflammatory mediators, which explains how this peptide is involved in disease progression. Nevertheless, apelin levels in patients with RA were lower than in healthy controls.166 Apelin is a determining element in OA progression, increasing the mRNA expression of several metalloproteinases, such as MMP1, MMP3, and MMP9, and IL1b in vitro.167 By contrast, intra-articular injection of apelin decreased levels of type II collagen, ADAMTS4 and ADAMTS5.167–168 Some studies have reported higher levels of apelin in patients with OA than in healthy controls.168 www.drugdiscoverytoday.com 9 KEYNOTE Drug Discovery Today d Volume 27, Number 11 d November 2022 KEYNOTE Drug Discovery Today FIGURE 5 Schematic of the role of chemerin in bone. Chemerin decreases osteoblast generation, induces osteoclast differentiation, and induces bone degeneration. It also contributes to disk degradation by reducing the synthesis of matrix proteins and inducing the synthesis of metalloproteinases. Vaspin Vaspin is a protein excreted by WAT with an important role in metabolic diseases, such as obesity or diabetes.169 This adipokine was identified for the first time in the visceral adipose tissue of the type 2 diabetes model Otsuka Long–Evans Tokushima Fatty rat, being part of the serpin family, explaining why it is also called serpinA12.170 Vaspin protein and gene expression were first detected in visceral adipose tissue, and were notably higher in obese patients. This expression, observed in cartilage, synovium, meniscus, IPFP, and osteophytes, suggests that vaspin is involved in pathways related to OA.171 Higher serum levels of vaspin we re detected in patients with RA compared with controls.172 There is also evidence that levels of vaspin are higher in RA than OA in the synovial fluid.173 Vaspin has been reported to prevent osteoclastogenesis in RAW 264.7 cells by inhibiting nuclear factor of activated T cell c1 expression.174 Some data showed how serum levels of vaspin and BMD in femoral neck and hip are positively correlated in postmenopausal women.175 There is also a downregulation of the vaspin mRNA levels in PBMCs in patients with SLE, which suggests that this adipokine is important in disease progression, although more studies are required to confirm this.176 Progranulin Progranulin (PGRN) is a glycoprotein of 593 amino acids encoded by GRN. It was first identified as a growth factor in 1990, and has since been found to be involved in many biological and pathological processes, including inflammation, cancer, immunity, and diabetes, among others. It is secreted by several cell types, such as adipocytes, chondrocytes, macrophages, and 10 www.drugdiscoverytoday.com skeletal muscle cells. Full-length PGRN has 7.5 granulin domains, which can be released individually via proteolytic cleavage by many enzymes.177 PGRN binds to the TNF receptors TNFR1 and TNFR2, thus interfering with TNFa-mediated inflammatory signaling pathways.178 It also binds to death receptor 3 (DR3), which is involved in various inflammatory processes, inhibiting its binding to its only known ligand, TL1A.179 In addition, PGRN induces the proliferation of Tregs and the production of the anti-inflammatory cytokine IL10 via FOXO4–STAT3.180 Many studies have confirmed the anti-inflammatory role of PGRN in RA and OA. PGRN levels in serum and synovial fluids of patients with RA were higher than in healthy controls.181–182 Moreover, the PGRN/TNFa ratio was correlated to the stage of the disease. Recently, serum PGRN levels have been suggested as a promising indicator of postoperative disease activity in patients with RA who underwent orthopedic surgery.183 In patients with OA, the expression of PGRN protein and mRNA was increased in cartilage, synovial, and IPFP tissue samples.184 In addition to its anti-inflammatory action, PGRN showed a chondroprotective role in OA by promoting the anabolism of chondrocytes mainly through the TNFR2/AKT pathway.185 Similar to RA and OA, the PGRN levels in patients with SLE were significantly higher and correlated with disease activity,78,186 although its role in the disease progression remains controversial.187 The previously mentioned susceptibility of PGRN to proteolytic cleavage hinders its use as a therapeutic agent. The individually released granulins are believed to be proinflammatory and can counteract the anti-inflammatory action of full-length PGRN. The granulin domains F, A, and C, as well as the adjacent linker region, were identified as domains involved in binding Drug Discovery Today d Volume 27, Number 11 d KEYNOTE November 2022 What’s new? Meteorin-like protein Meteorin-like (Metrnl) (also known as IL41) is a novel cytokine expressed by activated macrophages and barrier tissues.192 Although some cytokines, such as TNFa, IL17a, IL12, and IL4, induce the production of Metrnl by bone marrow macrophages, others, such as IFNc and TGFb, inhibit that production. The levels of Metrnl in circulation were reported to be related to in vivo inflammatory responses.192 Over the past few years, it became clear that Metrnl is associated with some human diseases in addition to being overexpressed in psoriasis and RA. These KEYNOTE with TNF receptors. This information gave rise to the development of atsttrin, a more stable PGRN-derived engineered molecule comprising half units of granulins F, A,and C, in addition to the linker region.188 Atsttrin binds specifically to TNF receptors and was proved to be even more potent in terms of its anti-inflammatory effects than PGRN itself.179,189 It was also reported to have a protective role in OA,177 bone regeneration activity,190 and, more recently, as a therapeutic agent for IVDD in mice.191 Despite these promising data, there is a lack of research around atsttrin, with no reported clinical trials as yet. (See Table 1-6). TABLE 1 Recent studies involving adipokines in OA. Protein Model of study Conclusion Refs Leptin Human serum OA chondrocytes Human serum Synovial fluid OA sinoviocytes OA synovial fibroblast Human synovia RNA from cartilage Human cartilage Increased in patients with knee OA High doses induce cell senescence mediated by mTOR pathway Higher in patients with OA and increase with clinical stage Correlates with severity and local inflammation in knee OA in women MiRNA might mediate oxidative stress produced by NF-jB induction by visfatin and resistin Promotes IL-6 and TNFa production by the inhibition of miR199a-5p expression Induces ICAM-1 expression and monocyte adhesion by inhibiting miR-320a Increases matrix mineralization and reduces collagen type I expression Promotes degradation of hip OA cartilage proteoglycan and induces production of proinflammatory cytokines (IL-6, MCP-1, CCL20, and CCL4) and MMPs Correlates with knee OA progression and inflammation state Correlates with OA; affects VCAM-1 expression and monocyte adhesion in human OASFs by inhibiting miR-381 Enhances expression of TNF-a and IL-1b in OASFs by inhibiting miR-149 expression Overexpression and knockout do not affect OA pathogenesis Synovial fluid and synovial membrane levels higher in patients with OA 13 Correlated with knee, hip, and wrist OA Significantly enhances phosphorylation of AKT/ERK in chondrocytes Levels positively correlated with cartilage destruction Serum and synovia levels correlated with cartilage degradation 145 Leptin and resistin Adiponectin Visfatin and resistin Visfatin Resistin LCN2 Chemerin Adipsin Human serum Human serum and OASFs Mouse Human serum and synovial fluid Human serum OA rat chondrocytes Human serum Human serum, synovia, and cartilage 200 14 54 99 100 101 93 102 117 118 119 133 114 146 144 159 TABLE 2 Recent studies involving adipokines in RA. Protein Model of study Conclusion Refs Leptin Leptin and resistin RA synovial fibroblasts Human serum 11 Leptin and vaspin [Adi’]ponectin Human serum RA synovial fibroblasts Human serum and mouse Increases ROS expression trigger RA-FLS migration Resistin concentrations correlate with radiographic progression; leptin promotes new bone formation in AS Levels increase in early RA Inhibition of p38 MAPK or AMPK is not sufficient to inhibit adiponectin signal Induces expression of OPN, which recruits osteoclasts and initiates bone erosion in patients with RA Induces a proinflammatory state in osteoblasts and osteoclasts Circulating adiponectin levels significantly higher in patients with RA than in controls High levels correlate with functional disability in RA Plasma levels correlate with disease activity Higher levels increase risk of moderate–severe disease activity in RA Contributes to pathogenesis of RA by increasing chemokine production by FLSs via CAP1 in synovial tissue High levels correlated with inflammation; increases TNFa production in macrophages 104 Serum levels correlate with RA and disease activity 129 Adiponectin and visfatin Chemerin Osteoclasts and osteoclasts Human serum Human serum Resistin FLS LCN2 Human serum and macrophages Human serum 31 153 45 46 74 52 140 141 113 115 www.drugdiscoverytoday.com 11 KEYNOTE Drug Discovery Today d Volume 27, Number 11 d November 2022 TABLE 3 Recent studies involving adipokines in AS. Protein Model of study Conclusion Refs Resistin, leptin, and adiponectin Leptin Leptin and resistin Human serum Human serum Human serum 30 LCN2 Leptin and adiponectin Human and mouse sera Human serum Only serum resistin is associated with AS development Correlates with AS stage Resistin concentrations correlated with radiographic progression; leptin promotes new bone formation in AS High levels associated with AS; PPARy agonist upregulates LCN2 Inversely correlate with radiographic progression 32 31 139 32 KEYNOTE TABLE 4 Recent studies involving adipokines in SLE. Protein Model of study Conclusion Refs Adiponectin, adipsin, and resistin Human serum 78 Adiponectin Human serum Adipsin and resistin are elevated in patients with SLE; adiponectin correlates with disease activity No association between adiponectin level and risk of SLE Correlates with SLE disease activity Leptin levels reduced in SLE and correlate negatively with disease stage; adiponectin levels are related to SLE and disease stage Both levels are related to SLE Vaspin levels decrease and adiponectin levels increase in SLE Leptin, adipsin, chemerin, and resistin Human serum Adiponectin and resistin Vaspin and adiponectin Human serum Human PBMCs 79 124 78 126 137 TABLE 5 Recent studies involving adipokines in osteoporosis. Protein Model of study Conclusion Refs Leptin Rat 19 Adiponectin Bone mesenchymal stem cell Overexpression induces osteogenic differentiation and can stimulated periodontal regeneration in osteoporotic conditions Inhibits osteoclastogenesis by suppressing NF-jB and MAPK pathways Facilitates osteogenic differentiation and osteogenesis by Wnt/b-catenin pathway modulation Inhibits osteoclast differentiation and proliferation of BMMs Does not correlate with BMD Levels do not change in patients with osteoporosis or osteoarthritis Inhibits osteoblastogenic signals mediated by WNT pathway Increases osteoclast activity and have crucial role in bone homeostasis 90 Promotes osteogenic differentiation and bone formation via Akt/Gsk3b/b-catenin axis Lower in patients with osteoporosis than in healthy patients Correlate with BMD 150 Bone marrow-derived monocytes Human serum Human serum MSCs from rat Mouse and bone marrow cells from mouse Bone marrow cells Visfatin LCN2 Chemerin Apelin Adiponectin and resistin Human serum Human serum 70 71 53 132 147 148 164 160 TABLE 6 Recent studies involving adipokines in IVD Degeneration. Protein Model of study Conclusion Refs Visfatin Resistin Rat model macrophages 110 Chemerin Human and rat sera Promotes IL-6 expression in NP cells via JNK/ERK/p38-MAPK signaling pathways Increases expression of CCL4 through p38-MAPK and NF-jB signaling pathways in NP cells, causing infiltration of macrophages Levels higher in degenerated nucleus pulposus tissues; overexpression related to IVDD progression in rat; induces matrix degradation by NF-jB signaling 12 www.drugdiscoverytoday.com 123 151 d Volume 27, Number 11 d KEYNOTE November 2022 data suggest that Metrnl has a significant role in chronic inflammatory diseases.193 Asprosin The novel adipokine asprosin was discovered in 2016 in a study of Neonatal Progeroid Syndrome (NPS),194 in which patients have a mutation of FBN1, and a lack of asprosin. This adipokine is derived by the cleavage of profibrilin 1, encoded by the last two exons of this protein.195 This adipokine was shown to stimulate orexigenic agouti-related protein peptide neurons (AgRP1) and inhibit anorexigenic neurons proopiomelanocortin (POMC),194 regulating appetite. As an experimental outcome, it was described how recombinant asprosin induces hyperglycemia and hyperinsulinemia.196 Recently, asprosin was described to be localized in human cartilage, and its levels in serum to be correlated with the cartilage marker COMP upon hip replacement surgery in patients with OA.197 Isthmin 1 Recently, reports showed that adipocytes secrete a novel protein with insulin-like effects, called isthmin 1.198 A clear correlation was recently found between isthmin 1 and obesity in pubertal boys,199 suggesting isthmin 1 as a potential biomarker of obesity in young populations. However, this association was not present in young women, indicating a putative sexual dimorphism.199 Concluding remarks MSDs are one of the major causes of disability around the world. To decrease their burden and improve the quality of life of patients, it is vital to identify novel biomarkers and new potential therapeutic targets. The dysfunction of adipose tissue, as in obesity, has emerged as a relevant link between metabolic alterations and MSDs. Adipokines constitute a superfamily of bioactive molecules, which have been extensively studied over the past 25 years and have a key role in MSDs. Most of these molecules have been reported to be associated with MSDs, and most are produced by musculoskeletal cells, such as chondrocytes, synovial cells, or bone cells, promoting a metabolic imbalance and triggering or cooperating in amplifying an inflammatory response. This suggests that the local production of adipokines in the musculoskeletal system has an additional role in immune–metabolic crosstalk. Clarifying the roles and mechanisms driving the functionality of adipokines in MSDs is crucial, not only for understanding the pathophysiology of the diseases themselves, but also for the identification of new therapeutic targets and the design of novel therapeutic molecules. A clear example of this approach is the identification of the binding domains in PGRN that allow binding to TNFR2, which gave rise to the engineering of atsttrin, a more stable and specific PGRNderived molecule with promising anti-inflammatory, chondroprotective, and bone regenerative properties.177 It is challenging to fully identify the contribution of each adipokine in the pathogenesis of inflammatory, metabolic, and immuno-associated diseases because most adipokines show wide range of functionalities across different tissues and cell types. Hence, there are only a few approved pharmacologically active molecules with adipokines as therapeutic targets. Some of these molecules are primarily aimed at targeting adipokine receptors, such as the recombinant methionyl human leptin (metreleptin) and leptin itself, whereas other drugs have been repurposed as regulators or modulators of adipokine secretion, as in the case of thiazolidinediones and metformin. In terms of leptin, probably the a are nearing a clearest view as chondrodestructive role in OA, which could lead to potential avenues for a leptin-based therapy. For instance, the control of circulating leptin levels, as well as local joint levels, by means of soluble, high-affinity leptin-binding molecules, analogous to anti-TNFa biologicals used to treat RA, might be a feasible option. Another conceivable recourse might be the block of leptin receptors with specific monoclonal humanized antibodies or mutant leptins that are capable of binding to leptin receptors without activating them. It is clear that these molecules should be designed to avoid the block of leptin receptors in the central nervous system, which could induce obesity and hyperphagia. Nevertheless, new advances in drug bioengineering and drug delivery (e.g., the use of biodegradable injectable hydrogels) would suggest that such local therapy is possible. Therefore, it will be vital to better characterize the pathophysiological role of adipokines in preclinical models of musculoskeletal degenerative-inflammatory diseases. Unfortunately, data from preclinical studies are scarce and support no conclusions as yet. Therefore, it is of great interest to evaluate these molecules in MSDs in which adipokines are involved. In addition, as more information about the roles of adipokines in MSDs becomes available, these molecules are likely to be crucial for the development of novel pharmacological approaches to immune-inflam matory-degenerative MSDs. Data availability No data was used for the research described in the article. Acknowledgments This work was supported by Xunta de Galicia (Servizo Galego de Saude, SERGAS), through a research-staff contract (ISCIII/SERGAS) to O.G, which is SERGAS Staff Personnel (I3SNS stable Researchers). Instituto de Salud Carlos III (ISCIII) and FEDER funded through a predoctoral research scholar to C.R-F. (Exp.18/00188). M.G-R. is a recipient of predoctoral contract funded by Xunta de Galicia (IN606A-2020/010). A.C.B. is a recipient of a predoctoral contract funded by Secretaría de Estado de Universidades, Investigación, Desarrollo e Innovación, Ministerio de Universidades (FPU2018- 04165). Y.F is a ‘Sara Borrell’ researcher funded by ISCIII and FEDER [CD21/00042]. O.G. is member of Red de Inflamación e Inmunopatología de Órganos y Sistemas - Enfermedades Inflamatorias [RD21/ 0002/0025] via ISCIII and FEDER. ISCIII and FEDER also supports O.G. and J.P. [ PI20/00902]. The work of O.G. was also supported by Research Executive Agency of the European Union in the framework of MSCA-RISE Action of the H2020 Program [Project number 734899], and Xunta de Galicia, Consellería de Educación, Universidade e Formación Profesional and Consellería de Economía, Emprego e Industria (GAIN) [GPC IN607B2019/10]. We would like to acknowledge Dr. Miguel-Angel González-Gay, Dr. Antonio Mera, Dr. Francisca Lago, Dr. Maurizio Capuozzo, and www.drugdiscoverytoday.com 13 KEYNOTE Drug Discovery Today KEYNOTE Miss Mariam Farrag for their help and valuable discussions during the writing of this article. Drug Discovery Today d Volume 27, Number 11 d November 2022 Declaration of interests. The authors declare no conflict of interest. References KEYNOTE [1] L. Recinella, G. Orlando, C. Ferrante, A. Chiavaroli, L. Brunetti, S. Leone, Adipokines: new potential therapeutic target for obesity and metabolic, rheumatic, and cardiovascular diseases, Front Physiol 11 (2020) 578966. [2] A. Cieza, K. Causey, K. Kamenov, S.W. Hanson, S. Chatterji, T. Vos, Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: a systematic analysis for the Global Burden of Disease Study 2019, Lancet 396 (2020) 2006–2017. [3] H. Masuzaki, Y. Ogawa, K. Hosoda, et al., Glucocorticoid regulation of leptin synthesis and secretion in humans: elevated plasma leptin levels in Cushing’s syndrome, J Clin Endocrinol Metab 82 (1997) 2542–2547. [4] J.W. Kolaczynski, M.R. Nyce, R.V. Considine, G. Boden, J.J. Nolan, R. Henry, et al., Acute and chronic effect of insulin on leptin production in humans: studies in vivo and in vitro, Diabetes 45 (1996) 699–701. [5] J.N. Fain, C.W. Leffler, J. Cowan, C. Buffington, L. Pouncey, S.W. Bahouth, Stimulation of leptin release by arachidonic acid and prostaglandin E2 in adipose tissue from obese humans, Metabolism 50 (2001) 921–928. [6] H. Shimizu, Y. Shimomura, Y. Nakanishi, T. Futawatari, K. Ohtani, N. Sato, et al., Estrogen increases in vivo leptin production in rats and human subjects, J Endocrinol 154 (1997) 285–292. [7] V. Francisco, C. Ruiz-Fernández, J. Pino, A. Mera, M.A. González-Gay, R. Gómez, et al., Adipokines: linking metabolic syndrome, the immune system, and arthritic diseases, Biochem Pharmacol 165 (March) (2019) 196–206. [8] K. Chihara, N. Hattori, N. Ichikawa, T. Matsuda, T. Saito, Re-evaluation of serum leptin and adiponectin concentrations normalized by body fat mass in patients with rheumatoid arthritis, Sci Rep 10 (2020) 15932. [9] J.A.D.J. Batún-Garrido, M. Salas-Magaña, I.E. Juárez-Rojop, Association between leptin and IL-6 concentrations with cardiovascular risk in patients with rheumatoid arthritis, Clin Rheumatol 37 (2018) 631–637. [10] S.Y. Oner, O. Volkan, C. Oner, A. Mengi, H. Direskeneli, D.A. Tasan, Serum leptin levels do not correlate with disease activity in rheumatoid arthritis, Acta Reumatologica Portuguesa 40 (2015) 50–54. [11] X. Sun, J. Wei, Y. Tang, B. Wang, Y. Zhang, L. Shi, et al., Leptin-induced migration and angiogenesis in rheumatoid arthritis is mediated by reactive oxygen species, FEBS Open Bio 7 (2017) 1899–1908. [12] A. Avdeeva, Y. Rubtsov, D. Dyikanov, T. Popkova, E. Nasonov, Regulatory T cells in patients with early untreated rheumatoid arthritis: Phenotypic changes in the course of methotrexate treatment, Biochimie 174 (2020) 9–17. [13] S. Min, T. Shi, X. Han, D. Chen, Z. Xu, D. Shi, et al., Serum levels of leptin, osteopontin, and sclerostin in patients with and without knee osteoarthritis, Clin Rheumatol 40 (2021) 287–294. [14] S.N. Lambova, T. Batsalova, D. Moten, S. Stoyanova, E. Georgieva, L. BelenskaTodorova, et al., Serum leptin and resistin levels in knee osteoarthritis—clinical and radiologic links: towards precise definition of metabolic type knee osteoarthritis, Biomedicines 9 (2021) 1019. [15] A. Cordero-Barreal, M. González-Rodríguez, C. Ruiz-Fernández, D.A. Eldjoudi, Y.R.F. AbdElHafez, F. Lago, et al., An update on the role of leptin in the immuno-metabolism of cartilage, Int J Mol Sci 22 (2021) 1–16. [16] X. Zhao, P. Huang, G. Li, L. Zhendong, G. Hu, Q. Xu, Activation of the leptin pathway by high expression of the long form of the leptin receptor (Ob-Rb) accelerates chondrocyte senescence in osteoarthritis, Bone Jt Res 8 (2019) 425– 436. [17] B. Liu, Y.H. Gao, N. Dong, C.W. Zhao, Y.F. Huang, J.G. Liu, et al., Differential expression of adipokines in the synovium and infrapatellar fat pad of osteoarthritis patients with and without metabolic syndrome, Connect Tissue Res 60 (2019) 611–618. [18] B. Raud, C. Gay, C. Guiguet-Auclair, A. Bonnin, L. Gerbaud, B. Pereira, et al., Level of obesity is directly associated with the clinical and functional consequences of knee osteoarthritis, Sci Rep 10 (2020) 1–7. [19] B. Zheng, J. Jiang, Y. Chen, M. Lin, Z. Du, Y. Xiao, et al., Leptin overexpression in bone marrow stromal cells promotes periodontal regeneration in a rat model of osteoporosis, J Periodontol 88 (2017) 808–818. [20] W.S. Lian, R.W. Wu, Y.S. Chen, et al., MicroRNA-29a in osteoblasts represses high-fat diet-mediated osteoporosis and body adiposis through targeting leptin, Int J Mol Sci 22 (2021) 9135. [21] A.H. Segar, J.C.T. Fairbank, J. Urban, Leptin and the intervertebral disc: a biochemical link exists between obesity, intervertebral disc degeneration and low back pain—an in vitro study in a bovine model, Eur Spine J 28 (2019) 214–223. 14 www.drugdiscoverytoday.com [22] X. Li, X. Liu, Y. Wang, F. Cao, Z. Chen, Z. Hu, et al., Intervertebral disc degeneration in mice with type II diabetes induced by leptin receptor deficiency, BMC Musculoskelet Disord 21 (2020) 77. [23] D.M. Natelson, A. Lai, D. Krishnamoorthy, R.C. Hoy, J.C. Iatridis, S. IllienJünger, Leptin signaling and the intervertebral disc: Sex dependent effects of leptin receptor deficiency and Western diet on the spine in a type 2 diabetes mouse model, PLoS ONE 15 (2020) e0227527. [24] A.E.M.A. Afifi, R.M. Shaat, O.M. Gharbia, M. Elhanafy, A.S.G. Hasan, Role of serum leptin levels and leptin receptor gene polymorphisms in systemic lupus erythematosus, Clin Rheumatol 39 (2020) 3465–3472. [25] Y.H. Lee, G.G. Song, Association between circulating leptin levels and systemic lupus erythematosus: an updated meta-analysis, Lupus 27 (2018) 428–435. [26] X. Wang, Y. Qiao, L. Yang, S. Song, Y. Han, Y. Tian, et al., Leptin levels in patients with systemic lupus erythematosus inversely correlate with regulatory T cell frequency, Lupus 26 (2017) 1401–1406. [27] H. Chen, J. Qi, T. Liu, M. Zou, Y. Hu, J. Wei, et al., Leptin accelerates B cell dysfunctions via activating JAK/STAT3/5 and ERK1/2 pathways in patients with systemic lupus erythematosus, Clin Exp Rheumatol (2022), https://doi. org/10.55563/clinexprheumatol/84syjo. [28] E.V. Lourenço, A. Liu, G. Matarese, A. La Cava, Leptin promotes systemic lupus erythematosus by increasing autoantibody production and inhibiting immune regulation, Proc Natl Acad Sci U S A 113 (2016) 10637–10642. [29] Y.J. Mei, P. Wang, L.J. Chen, Z.J. Li, Plasma/serum leptin levels in patients with ankylosing spondylitis: a systematic review and meta-analysis, Arch Med Res 47 (2016) 111–117. [30] J. Yang, X. Zhang, Y. Ma, M. Wu, X. Hu, R. Han, et al., Serum levels of leptin, adiponectin and resistin in patients with ankylosing spondylitis: a systematic review and meta-analysis, Int Immunopharmacol 52 (July) (2017) 310–317. [31] J.H. Park, S.G. Lee, Y.K. Jeon, E.K. Park, Y.S. Suh, H.O. Kim, Relationship between serum adipokine levels and radiographic progression in patients with ankylosing spondylitis: a preliminary 2-year longitudinal study, Med (United States) 96 (2017) e7854. [32] A. Hartl, J. Sieper, U. Syrbe, J. Listing, K.G. Hermann, M. Rudwaleit, et al., Serum levels of leptin and high molecular weight adiponectin are inversely associated with radiographic spinal progression in patients with ankylosing spondylitis: results from the ENRADAS trial, Arthritis Res Ther 19 (2017) 140. [33] H.M. Choi, H.M. Doss, K.S. Kim, Multifaceted physiological roles of adiponectin in inflammation and diseases, Int J Mol Sci 21 (2020) 1219. [34] T. Nicholson, C. Church, D.J. Baker, S.W. Jones, The role of adipokines in skeletal muscle inflammation and insulin sensitivity, J Inflamm 15 (2018) 1–11. [35] S. Aleidi, A. Issa, H. Bustanji, M. Khalil, Y. Bustanji, Adiponectin serum levels correlate with insulin resistance in type 2 diabetic patients, Saudi Pharm J 23 (2015) 250–256. [36] T. Kadowaki, T. Yamauchi, Adiponectin and adiponectin receptors, Endocr Rev 26 (2005) 439–451. [37] J.Y. Myeong, Y.L. Gha, J.J. Chung, H.A. Young, H.H. Seung, B.K. Jae, Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor a, Diabetes 55 (2006) 2562–2570. [38] T. Yamauchi, J. Kamon, Y. Minokoshi, Y. Ito, H. Waki, S. Uchida, et al., Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase, Nat Med 8 (2002) 1288–1295. [39] A. Davoudi-Kiakalayeh, R. Mohammadi, A.A. Pourfathollah, Z. Siery, S. Davoudi-Kiakalayeh, Alloimmunization in thalassemia patients: new insight for healthcare, Int J Prev Med 8 (2017) 101. [40] H. Ruan, L.Q. Dong, Adiponectin signaling and function in insulin target tissues, J Mol Cell Biol 8 (2016) 101–109. [41] D. Azamar-Llamas, G. Hernández-Molina, B. Ramos-àvalos, J. FuruzawaCarballeda, Adipokine contribution to the pathogenesis of osteoarthritis, Mediators Inflamm 2017 (2017) 5468023. [42] K. Ohashi, J.L. Parker, N. Ouchi, A. Higuchi, J.A. Vita, N. Gokce, et al., Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype, J Biol Chem 285 (2010) 6153–6160. [43] N. Kusunoki, K. Kitahara, F. Kojima, N. Tanaka, K. Kaneko, H. Endo, et al., Adiponectin stimulates prostaglandin E2 production in rheumatoid arthritis synovial fibroblasts, Arthritis Rheum 62 (2010) 1641–1649. d Volume 27, Number 11 d November 2022 KEYNOTE [44] C.H. Tang, Y.C. Chiu, T.W. Tan, R.S. Yang, W.M. Fu, Adiponectin enhances IL6 production in human synovial fibroblast via an AdipoR1 receptor, AMPK, p38, and NF-jB pathway, J Immunol 179 (2007) 5483–5492. [45] K. Khawaja, K.W. Frommer, M. Bausch, S. Rehart, U. Müller-Ladner, E. Neumann, Compensation of adiponectin-induced adenosine monophosphate-activated protein kinase and p38 mitogen-activated protein kinase signaling in rheumatoid arthritis synovial fibroblasts, J Interf Cytokine Res 41 (2021) 177–186. [46] J. Qian, L. Xu, X. Sun, Y. Wang, W. Xuan, Q. Zhang, et al., Adiponectin aggravates bone erosion by promoting osteopontin production in synovial tissue of rheumatoid arthritis, Arthritis Res Ther 20 (2018) 1–11. [47] J. Mohiti-Ardekani, H. Soleymani-Salehabadi, M.B. Owlia, A. Mohiti, Relationships between serum adipocyte hormones (adiponectin, leptin, resistin), bone mineral density and bone metabolic markers in osteoporosis patients, J Bone Miner Metab 32 (2014) 400–404. [48] K. Kishida, T. Funahashi, I. Shimomura, Adiponectin as a routine clinical biomarker, Best Pract Res Clin Endocrinol Metab 28 (2014) 119–130. [49] M. Otero, R. Lago, R. Gomez, F. Lago, C. Dieguez, J.J. Gómez-Reino, et al., Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis, Ann Rheum Dis 65 (2006) 1198–1201. [50] C. Bustos Rivera-Bahena, D.X. Xibillé-Friedmann, J. González-Christen, S.M. Carrillo-Vázquez, J.L. Montiel-Hernández, Peripheral blood leptin and resistin levels as clinical activity biomarkers in Mexican rheumatoid arthritis patients, Reumatol Clínica 12 (2016) 323–326. [51] S. Chennareddy, K.V. Kishore Babu, S. Kommireddy, R. Varaprasad, L. Rajasekhar, Serum adiponectin and its impact on disease activity and radiographic joint damage in early rheumatoid arthritis - a cross-sectional study, Indian J Rheumatol 11 (2016) 82–85. [52] Y.H. Lee, S.C. Bae, Circulating adiponectin and visfatin levels in rheumatoid arthritis and their correlation with disease activity: a meta-analysis, Int J Rheum Dis 21 (2018) 664–672. [53] X. Wu, L. Huang, J. Liu, Effects of adiponectin on osteoclastogenesis from mouse bone marrow-derived monocytes, Exp Ther Med 17 (2018) 1228–1233. [54] C. Orellana, J. Calvet, A. Berenguer-Llergo, N. Albiñana, M. García Manrique, C. Galisteo Lencastre, et al., Synovial adiponectin was more associated with clinical severity than synovial leptin in women with knee osteoarthritis, Cartilage 13 (2021) 1675S–1683S. [55] E. Distel, T. Cadoudal, S. Durant, A. Poignard, X. Chevalier, C. Benelli, The infrapatellar fat pad in knee osteoarthritis: an important source of interleukin-6 and its soluble receptor, Arthritis Rheum 60 (2009) 3374–3377. [56] R. Lago, R. Gomez, M. Otero, F. Lago, R. Gallego, C. Dieguez, et al., A new player in cartilage homeostasis: adiponectin induces nitric oxide synthase type II and pro-inflammatory cytokines in chondrocytes, Osteoarthr Cartil 16 (2008) 1101–1109. [57] E.H. Kang, Y.J. Lee, T.K. Kim, C.B. Chang, J.H. Chung, K. Shin, et al., Adiponectin is a potential catabolic mediator in osteoarthritis cartilage, Arthritis Res Ther 12 (2010) R231. [58] Q. Wang, J. Cai, J. Wang, C. Xiong, L. Yan, Z. Zhang, et al., Down-regulation of adiponectin receptors in osteoarthritic chondrocytes, Cell Biochem Biophys 70 (2014) 491–497. [59] Y. Yao, Y. Wang, ATDC5: an excellent in vitro model cell line for skeletal development, J Cell Biochem 114 (2013) 1223–1229. [60] T.D. Challa, Y. Rais, E.M. Ornan, Effect of adiponectin on ATDC5 proliferation, differentiation and signaling pathways, Mol Cell Endocrinol 323 (2010) 282– 291. [61] A. Askari, P. Arasteh, R. Homayounfar, M.M. Naghizadeh, E. Ehrampoush, S.M. Mousavi, et al., The role of adipose tissue secretion in the creation and pain level in osteoarthritis, Endocr Regul 54 (2020) 6–13. [62] P.J. Francin, A. Abot, C. Guillaume, D. Moulin, A. Bianchi, P. Gegout-Pottie, et al., Association between adiponectin and cartilage degradation in human osteoarthritis, Osteoarthr Cartil 22 (2014) 519–526. [63] C. Korkmaz, Response to ‘Adiponectin associates with markers of cartilage degradation in osteoarthritis and induces production of proinflammatory and catabolic factors through mitogen-activated protein kinase pathways’, Arthritis Res Ther 14 (2012) 402. [64] T.H. Chen, L. Chen, M.S. Hsieh, C.P. Chang, D.T. Chou, S.H. Tsai, Evidence for a protective role for adiponectin in osteoarthritis, Biochim Biophys Acta - Mol Basis Dis 1762 (2006) 711–718. [65] C.H. Te, H.K. Tsou, J.C. Chen, J.M.K. Shih, Y.J. Chen, C.H. Tang, Adiponectin enhances intercellular adhesion molecule-1 expression and promotes monocyte adhesion in human synovial fibroblasts, PLoS ONE 9 (2014) 1–10. KEYNOTE [66] H.S. Berner, S.P. Lyngstadaas, A. Spahr, M. Monjo, L. Thommesen, C.A. Drevon, et al., Adiponectin and its receptors are expressed in bone-forming cells, Bone 35 (2004) 842–849. [67] J. Jürimäe, K. Rembel, T. Jürimäe, M. Rehand, Adiponectin is associated with bone mineral density in perimenopausal women, Horm Metab Res 37 (2005) 297–302. [68] G. Iacobellis, M. Iorio, N. Napoli, D. Cotesta, L. Zinnamosca, C. Marinelli, et al., Relation of adiponectin, visfatin and bone mineral density in patients with metabolic syndrome, J Endocrinol Invest 34 (2011) e12–e15. [69] X.H. Luo, L.J. Guo, H. Xie, L.Q. Yuan, X.P. Wu, H.D. Zhou, et al., Adiponectin stimulates RANKL and inhibits OPG expression in human osteoblasts through the MAPK signaling pathway, J Bone Miner Res 21 (2006) 1648–1656. [70] G. Chen, L. Huang, X. Wu, X. Liu, Q. Xu, F. Li, et al., Adiponectin inhibits osteoclastogenesis by suppressing NF-jB and p38 signaling pathways, Biochem Biophys Res Commun 503 (2018) 2075–2082. [71] Y. Wang, X. Zhang, J. Shao, H. Liu, X. Liu, E. Luo, Adiponectin regulates BMSC osteogenic differentiation and osteogenesis through the Wnt/b-catenin pathway, Sci Rep 7 (2017) 1–13. [72] X. Bi, Y.T. Loo, C.J. Henry, Relationships between adiponectin and bone: sex difference, Nutrition 70 (2020) 110489. [73] C.M. Su, W.L. Lee, C.J. Hsu, T.T. Lu, L.H. Wang, G.H. Xu, et al., Adiponectin induces oncostatin M expression in osteoblasts through the PI3K/Akt signaling pathway, Int J Mol Sci 17 (2016) 29. [74] G. Krumbholz, S. Junker, F.M.P. Meier, M. Rickert, J. Steinmeyer, S. Rehart, et al., Response of human rheumatoid arthritis osteoblasts and osteoclasts to adiponectin, Clin Exp Rheumatol 35 (2017) 406–414. [75] U. Syrbe, J. Callhoff, K. Conrad, D. Poddubnyy, H. Haibel, S. Junker, et al., Serum adipokine levels in patients with ankylosing spondylitis and their relationship to clinical parameters and radiographic spinal progression, Arthritis Rheumatol 67 (2015) 678–685. [76] T.P. Zhang, H.M. Li, R. Li, Q. Zhang, Y.G. Fan, X.M. Li, et al., Association of omentin-1, adiponectin, and resistin genetic polymorphisms with systemic lupus erythematosus in a Chinese population, Int Immunopharmacol 83 (2020) 106343. [77] B.V. de Souza, P.L. Francescantônio, N.A. da Silva, Leptina e adiponectina no lúpus eritematoso sistêmico: Correlações clínicas e laboratoriais, Rev Bras Reumatol 55 (2015) 140–145. [78] D. Chougule, M. Nadkar, K. Venkataraman, A. Rajadhyaksha, N. Hase, T. Jamale, et al., Adipokine interactions promote the pathogenesis of systemic lupus erythematosus, Cytokine 111 (March) (2018) 20–27. [79] Y.L. Dan, P. Wang, Z. Cheng, Q. Wu, X.R. Wang, D.G. Wang, et al., Circulating adiponectin levels and systemic lupus erythematosus: a two-sample Mendelian randomization study, Rheumatology 60 (2021) 940–946. [80] B. Samal, Y. Sun, G. Stearns, C. Xie, S. Suggs, I. McNiece, Cloning and characterization of the cDNA encoding a novel human pre-B-cell colonyenhancing factor, Mol Cell Biol 14 (1994) 1431–1437. [81] A. Fukuhara, M. Matsuda, M. Nishizawa, K. Segawa, M. Tanaka, K. Kishimoto, et al., Visfatin: a protein secreted by visceral fat that Mimics the effects of insulin, Science (80-) 307 (2005) 426–430. [82] D. Friebe, M. Neef, J. Kratzsch, S. Erbs, K. Dittrich, A. Garten, et al., Leucocytes are a major source of circulating nicotinamide phosphoribosyltransferase (NAMPT)/pre-B cell colony (PBEF)/visfatin linking obesity and inflammation in humans, Diabetologia 54 (2011) 1200–1211. [83] A. Dakroub, S. Nasser, N. Younis, H. Bhagani, Y. Al-Dhaheri, G. Pintus, et al., Visfatin : a possible role in cardiovasculo-metabolic disorders, Cells (2020:) 244. [84] Y.J. Heo, S.E. Choi, J.Y. Jeon, et al., Visfatin induces inflammation and insulin resistance via the NF-jB and STAT3 signaling pathways in hepatocytes, J Diabetes Res (2019; 2019.). [85] H. Xie, S.Y. Tang, X.H. Luo, J. Huang, R.R. Cui, L.Q. Yuan, et al., Insulin-like effects of visfatin on human osteoblasts, Calcif Tissue Int 80 (2007) 201–210. [86] C. Jacques, M. Holzenberger, Z. Mladenovic, et al., Proinflammatory actions of visfatin/nicotinamide phosphoribosyltransferase (Nampt) involve regulation of insulin signaling pathway and Nampt enzymatic activity, J Biol Chem 287 (2012) 15100–15108. [87] R.R. Yammani, R.F. Loeser, Extracellular nicotinamide phosphoribosyltransferase (NAMPT/visfatin) inhibits insulin-like growth factor-1 signaling and proteoglycan synthesis in human articular chondrocytes, Arthritis Res Ther 14 (2012) 1–8. ski, Ł. Pilaczyn ska-Szczesniak, Bone [88] E. Sliwicka, A. Nowak, W. Zep, P. Leszczyn mass and bone metabolic indices in male master rowers, J Bone Miner Metab 33 (2015) 540–546. www.drugdiscoverytoday.com 15 KEYNOTE Drug Discovery Today KEYNOTE KEYNOTE [89] A.A. Siviero-Miachon, A.M. Spinola-Castro, M.L. de Martino Lee, et al., Visfatin is a positive predictor of bone mineral density in young survivors of acute lymphocytic leukemia, J Bone Miner Metab 35 (2017) 73–82. [90] G. Mihai, A.I. Gasparik, I.M. Pascanu, M. Cevei, A. Hutanu, R.M. Pop, The influence of Visfatin, RBP-4 and insulin resistance on bone mineral density in women with treated primary osteoporosis, Aging Clin Exp Res 31 (2019) 889–895. [91] S. Terzoudis, N. Malliaraki, J. Damilakis, D.A. Dimitriadou, C. Zavos, I.E. Koutroubakis, Chemerin, visfatin, and vaspin serum levels in relation to bone mineral density in patients with inflammatory bowel disease, Eur J Gastroenterol Hepatol 28 (2016) 814–819. [92] X. He, J. He, Y. Shi, C. Pi, Y. Yang, Y. Sun, et al., Nicotinamide phosphoribosyltransferase (Nampt) may serve as the marker for osteoblast differentiation of bone marrow-derived mesenchymal stem cells, Exp Cell Res 352 (2017) 45–52. [93] L. Tsiklauri, J. Werner, M. Kampschulte, K.W. Frommer, L. Berninger, M. Irrgang, et al., Visfatin alters the cytokine and matrix-degrading enzyme profile during osteogenic and adipogenic MSC differentiation, Osteoarthr Cartil 26 (2018) 1225–1235. [94] M. Ling, P. Huang, S. Islam, D.P. Heruth, X. Li, L.Q. Zhang, et al., Epigenetic regulation of Runx2 transcription and osteoblast differentiation by nicotinamide phosphoribosyltransferase, Cell Biosci 7 (2017) 1–10. [95] A.R. Moschen, S. Geiger, R. Gerner, H. Tilg, Pre-B cell colony enhancing factor/ NAMPT/visfatin and its role in inflammation-related bone disease, Mutat Res Fundam Mol Mech Mutagen 690 (2010) 95–101. [96] J.M. Baek, S.J. Ahn, Y.H. Cheon, M.S. Lee, J. Oh, J.Y. Kim, Nicotinamide phosphoribosyltransferase inhibits receptor activator of nuclear factor-jB ligand-induced osteoclast differentiation in vitro, Mol Med Rep 15 (2017) 784–792. [97] A.L. McNulty, M.R. Miller, S.K. O’Connor, F. Guilak, The effects of adipokines on cartilage and meniscus catabolism, Connect Tissue Res 52 (2011) 523–533. [98] R. Krysiak, G. Handzlik-Orlik, B. Okopien, The role of adipokines in connective tissue diseases, Eur J Nutr 51 (2012) 513–528. [99] S. Cheleschi, I. Gallo, M. Barbarino, S. Giannotti, N. Mondanelli, A. Giordano, et al., MicroRNA mediate visfatin and resistin induction of oxidative stress in human osteoarthritic synovial fibroblasts via NF-jB pathway, Int J Mol Sci 20 (2019) 5200. [100] M.H. Wu, C.H. Tsai, Y.L. Huang, Y.C. Fong, C.H. Tang, Visfatin promotes IL-6 and TNF-a production in human synovial fibroblasts by repressing miR-199a5p through ERK, p38 and JNK signaling pathways, Int J Mol Sci 19 (2018) 1–10. [101] Y.Y. Law, Y.M. Lin, S.C. Liu, M.H. Wu, W.H. Chung, C.H. Tsai, et al., Visfatin increases ICAM-1 expression and monocyte adhesion in human osteoarthritis synovial fibroblasts by reducing miR-320a expression, Aging (Albany NY) 12 (2020) 18635–18648. [102] A.M. Philp, S. Butterworth, E.T. Davis, S.W. Jones, Enampt is localised to areas of cartilage damage in patients with hip osteoarthritis and promotes cartilage catabolism and inflammation, Int J Mol Sci 22 (2021) 6719. [103] M.C. Laiguillon, X. Houard, C. Bougault, M. Gosset, G. Nourissat, A. Sautet, et al., Expression and function of visfatin (Nampt), an adipokine-enzyme involved in inflammatory pathways of osteoarthritis, Arthritis Res Ther 16 (2014) 1–12. [104] M.L. Vazquez-Villegas, J.I. Gamez-Nava, A.M. Saldaña-Cruz, A. Celis, E.N. Sanchez-Rodriguez, E.E. Perez-Guerrero, et al., Functional disability is related to serum chemerin levels in rheumatoid arthritis, Sci Rep 11 (2021) 1–8. [105] Y.M. Chen, P.K. Chen, C.K. Chang, C.C. Lin, H.H. Chen, J.L. Lan, et al., Association of apolipoprotein e polymorphism with adipokines and cardiovascular disease risk in rheumatoid arthritis patients, Life 10 (2020) 1– 15. [106] Z. Mirfeizi, Z. Noubakht, A.E. Rezaie, M.H. Jokar, Z.S. Sarabi, Plasma levels of leptin and visfatin in rheumatoid arthritis patients; is there any relationship with joint damage?, Iran J Basic Med Sci 17 (2014) 662–666 [107] X. Li, S. Islam, M. Xiong, N.N. Nsumu, M.W. Lee Jr, L.Q. Zhang, et al., Epigenetic regulation of NfatC1 transcription and osteoclastogenesis by nicotinamide phosphoribosyl transferase in the pathogenesis of arthritis, Cell Death Discov 5 (2019) 62. [108] E. Franco-Trepat, A. Alonso-Pérez, M. Guillán-Fresco, A. Jorge-Mora, O. Gualillo, J.J. Gómez-Reino, et al., Visfatin as a therapeutic target for rheumatoid arthritis, Expert Opin Ther Targets 23 (2019) 607–618. [109] D.M. Mohammed Ali, S.Z. Al-Fadhel, N.H.A. Al-Ghuraibawi, H.K. Al-Hakeim, Serum chemerin and visfatin levels and their ratio as possible diagnostic parameters of rheumatoid arthritis, Reumatologia 58 (2020) 67–75. [110] H. Cui, X. Du, C. Liu, S. Chen, H. Cui, H. Liu, et al., Visfatin promotes intervertebral disc degeneration by inducing IL-6 expression through the ERK/ JNK/p38 signalling pathways, Adipocyte 10 (2021) 201–215. 16 www.drugdiscoverytoday.com Drug Discovery Today d Volume 27, Number 11 d November 2022 [111] J.A. Miranda-Filloy, R. López-Mejias, F. Genre, B. Carnero-López, R. Ochoa, T. Diaz de Terán, et al., Leptin and visfatin serum levels in non-diabetic ankylosing spondylitis patients undergoing TNF-a antagonist therapy, Clin Exp Rheumatol 31 (2013) 538–545. [112] C. Yang, D. Tang, Patient-specific carotid plaque progression simulation, C Model Eng Sci 1 (2000) 119–131. [113] H. Sato, S. Muraoka, N. Kusunoki, S. Masuoka, S. Yamada, H. Ogasawara, et al., Resistin upregulates chemokine production by fibroblast-like synoviocytes from patients with rheumatoid arthritis, Arthritis Res Ther 19 (2017) 263. [114] H.M. Li, T.P. Zhang, X.M. Li, H.F. Pan, D.C. Ma, Association of single nucleotide polymorphisms in resistin gene with rheumatoid arthritis in a Chinese population, J Clin Lab Anal 32 (2018) e22595. [115] K. Vuolteenaho, L. Tuure, R. Nieminen, L. Laasonen, M. Leirisalo-Repo, E. Moilanen, et al., Pretreatment resistin levels are associated with erosive disease in early rheumatoid arthritis treated with disease-modifying anti-rheumatic drugs and infliximab, Scand J Rheumatol 51 (2022) 180–185. [116] G.K. Vasileiadis, A.C. Lundell, Y. Zhang, K. Andersson, I. Gjertsson, A. Rudin, et al., Adipocytokines in untreated newly diagnosed rheumatoid arthritis: association with circulating chemokines and markers of inflammation, Biomolecules 11 (2021) 1–13. [117] E.M. Alissa, L.S. Alzughaibi, Z.M. Marzouki, Relationship between serum resistin, body fat and inflammatory markers in females with clinical knee osteoarthritis, Knee 27 (2020) 45–50. [118] W.C. Chen, C.Y. Lin, S.J. Kuo, S.C. Liu, Y.C. Lu, Y.L. Chen, et al., Resistin enhances VCAM-1 expression and monocyte adhesion in human osteoarthritis synovial fibroblasts by inhibiting MiR-381 expression through the PKC, p38, and JNK signaling pathways, Cells 9 (2020) 1369. [119] W.C. Chen, Y.C. Lu, S.J. Kuo, C.Y. Lin, C.H. Tsai, S.C. Liu, et al., Resistin enhances IL-1b and TNF-a expression in human osteoarthritis synovial fibroblasts by inhibiting miR-149 expression via the MEK and ERK pathways, FASEB J 34 (2020) 13671–13684. [120] J. Zhu, G. Ruan, H. Cen, T. Meng, S. Zheng, Y. Wang, et al., Association of serum levels of inflammatory markers and adipokines with joint symptoms and structures in participants with knee osteoarthritis, Rheumatology 61 (2022) 1044–1052. [121] F.Y. Azizieh, D. Shehab, J.K. Al, O. Mojiminiyi, R. Gupta, R. Raghupathy, Circulatory pattern of cytokines, adipokines and bone markers in postmenopausal women with low BMD, J Inflamm Res 12 (2019) 99–108. [122] C. Liu, H. Yang, F. Gao, X. Li, Y. An, J. Wang, et al., Resistin promotes intervertebral disc degeneration by upregulation of ADAMTS-5 through p38 MAPK signaling pathway, Spine 41 (2016) 1414–1420. [123] Z. Li, X. Wang, H. Pan, H. Yang, X. Li, K. Zhang, et al., Resistin promotes CCL4 expression through toll-like receptor-4 and activation of the p38-MAPK and NF-jB signaling pathways: implications for intervertebral disc degeneration, Osteoarthr Cartil 25 (2017) 341–350. [124] M.A. Mahieu, G.E. Ahn, J.S. Chmiel, D.D. Dunlop, I.B. Helenowski, P. Semanik, et al., Serum adipokine levels and associations with patientreported fatigue in systemic lupus erythematosus, Rheumatol Int 38 (2018) 1053–1061. [125] C.Y. Kuo, T.Y. Tsai, Y.C. Huang, Insulin resistance and serum levels of adipokines in patients with systemic lupus erythematosus: a systematic review and meta-analysis, Lupus 29 (2020) 1078–1084. [126] J.I. Gamez-Nava, V. Diaz-Rizo, E.E. Perez-Guerrero, J.F. Muñoz-Valle, A.M. Saldaña-Cruz, N.S. Fajardo-Robledo, et al., Assessment of serum macrophage migration inhibitory factor (MIF), adiponectin, and other adipokines as potential markers of proteinuria and renal dysfunction in lupus nephritis: a cross-sectional study, Biomark Res 8 (2020) 55. [127] D. Apostolopoulos, F. Vincent, A. Hoi, E. Morand, Associations of metabolic syndrome in SLE, Lupus Sci Med 7 (2020) e000436. [128] A. Gigante, F. Iannazzo, L. Navarini, M.C. Sgariglia, D.P.E. Margiotta, V. Vaiarello, et al., Metabolic syndrome and adipokine levels in systemic lupus erythematosus and systemic sclerosis, Clin Rheumatol 40 (2021) 4253–4258. [129] A. Gulkesen, G. Akgol, A.K. Poyraz, S. Aydin, A. Denk, T. Yildirim, et al., Lipocalin 2 as a clinical significance in rheumatoid arthritis, Cent Eur J Immunol 42 (2017) 269–273. [130] C. Pirozzi, V. Francisco, F.D. Guida, R. Gómez, F. Lago, J. Pino, et al., Butyrate modulates inflammation in chondrocytes via GPR43 receptor, Cell Physiol Biochem 51 (2018) 228–243. [131] A. Villalvilla, A. García-Martín, R. Largo, O. Gualillo, G. Herrero-Beaumont, R. Gómez, The adipokine lipocalin-2 in the context of the osteoarthritic osteochondral junction, Sci Rep 6 (2016) 29243. [132] A. Maurizi, M. Ponzetti, K.M. Gautvik, S. Reppe, A. Teti, N. Rucci, Lipocalin 2 serum levels correlate with age and bone turnover biomarkers in healthy [133] [134] [135] [136] [137] KEYNOTE [138] [139] [140] [141] [142] [143] [144] [145] [146] [147] [148] [149] [150] [151] [152] [153] [154] d Volume 27, Number 11 d KEYNOTE November 2022 subjects but not in postmenopausal osteoporotic women, Bone Reports 14 (2021) 101059. W.S. Choi, J.S. Chun, Upregulation of lipocalin-2 (LCN2) in osteoarthritic cartilage is not necessary for cartilage destruction in mice, Osteoarthr Cartil 25 (2017) 401–405. Z. Jiang, X. Du, X. Wen, H. Li, A. Zeng, H. Sun, et al., Whole-transcriptome sequence of degenerative meniscus cells unveiling diagnostic markers and therapeutic targets for osteoarthritis, Front Genet 12 (2021) 754421. M. Ponzetti, A. Ucci, A. Maurizi, L. Giacchi, A. Teti, N. Rucci, Lipocalin 2 influences bone and muscle phenotype in the MDX mouse model of duchenne muscular dystrophy, Int J Mol Sci 23 (2022) 598. T.H. Kao, Y.J. Peng, D.M. Salter, H.S. Lee, Nerve growth factor increases MMP9 activity in annulus fibrosus cells by upregulating lipocalin 2 expression, Eur Spine J 24 (2015) 1959–1968. T.P. Zhang, H.M. Li, R.X. Leng, X.P. Li, X.M. Li, H.F. Pan, et al., Plasma levels of adipokines in systemic lupus erythematosus patients, Cytokine 86 (2016) 15– 20. J. Lindblom, C. Mohan, I. Parodis, Biomarkers in neuropsychiatric systemic lupus erythematosus: a systematic literature review of the last decade, Brain Sci 12 (2022) 192. A. Lin, R.D. Inman, C.J. Streutker, Z. Zhang, K.P.H. Pritzker, H.W. Tsui, et al., Lipocalin 2 links inflammation and ankylosis in the clinical overlap of inflammatory bowel disease (IBD) and ankylosing spondylitis (AS), Arthritis Res Ther 22 (2020) 51. B. Tolusso, M.R. Gigante, S. Alivernini, L. Petricca, A.L. Fedele, C. Di Mario, et al., Chemerin and PEDF are metaflammation-related biomarkers of disease activity and obesity in rheumatoid arthritis, Front Med 5 (2018) 7258152. F. Gonzalez-Ponce, J.I. Gamez-Nava, E.E. Gomez-Ramirez, M. RamirezVillafaña, H. Jacobo-Cuevas, N.A. Rodriguez-Jimenez, et al., Serum chemerin levels: a potential biomarker of joint inflammation in women with rheumatoid arthritis, PLoS ONE 16 (9 September) (2021) e0255854. M. Czókolyová, A. Pusztai, E. Végh, à. Horváth, A. Szentpéteri, A. Hamar, et al., Changes of metabolic biomarker levels upon one-year anti-TNF-a therapy in rheumatoid arthritis and ankylosing spondylitis: associations with vascular pathophysiology, Biomolecules 11 (2021) 1535. J. Ma, D.S. Niu, N.J. Wan, Y. Qin, C.J. Guo, Elevated chemerin levels in synovial fluid and synovial membrane from patients with knee osteoarthritis, Int J Clin Exp Pathol 8 (2015) 13393–13398. J. Martel-Pelletier, G. Tardif, J. Rousseau Trépanier, F. Abram, M. Dorais, J.P. Raynauld, et al., The ratio adipsin/MCP-1 is strongly associated with structural changes and CRP/MCP-1 with symptoms in obese knee osteoarthritis subjects: data from the Osteoarthritis Initiative, Osteoarthr Cartil 27 (2019) 1163–1173. L.J. Cajas Santana, F. Rondón Herrera, A.P. Rojas, D.J. Martínez Lozano, N. Prieto, C.M. Bohorquez, Serum chemerin in a cohort of Colombian patients with primary osteoarthritis, Reumatol Clin 17 (2021) 530–535. J. Ma, L. Ren, C.J. Guo, N.J. Wan, D.S. Niu, Chemerin affects the metabolic and proliferative capabilities of chondrocytes by increasing the phosphorylation of AKT/ERK, Eur Rev Med Pharmacol Sci 22 (2018) 3656–3662. S. Muruganandan, R. Govindarajan, N.M. McMullen, C.J. Sinal, Chemokinelike receptor 1 is a novel Wnt target gene that regulates mesenchymal stem cell differentiation, Stem Cells 35 (2017) 711–724. E.S. Ramos-Junior, G.A. Leite, C.C. Carmo-Silva, T.M. Taira, K.B. Neves, D.F. Colón, et al., Adipokine chemerin bridges metabolic dyslipidemia and alveolar bone loss in mice, J Bone Miner Res 32 (2017) 974–984. J. Li, L. Xiang, X. Jiang, B. Teng, Y. Sun, G. Chen, et al., Investigation of bioeffects of G protein-coupled receptor 1 on bone turnover in male mice, J Orthop Transl 10 (2017) 42–51. J. Li, T. Zhang, C. Huang, M. Xu, W. Xie, Q. Pei, et al., Chemerin located in bone marrow promotes osteogenic differentiation and bone formation via Akt/ Gsk3b/b-catenin axis in mice, J Cell Physiol 236 (2021) 6042–6054. S. Hu, Z. Shao, C. Zhang, L. Chen, A.A. Mamun, N. Zhao, et al., Chemerin facilitates intervertebral disc degeneration via TLR4 and CMKLR1 and activation of NF-kB signaling pathway, Aging (Albany NY) 12 (2020) 11732– 11753. N.J. Song, S. Kim, B.H. Jang, S.H. Chang, U.J. Yun, K.M. Park, et al., Small molecule-induced complement factor D (Adipsin) promotes lipid accumulation and adipocyte differentiation, PLoS ONE 11 (2016) e0162228. J. Rodríguez, G.I. Lafaurie, W. Bautista-Molano, L. Chila-Moreno, J.M. BelloGualtero, C. Romero-Sánchez, Adipokines and periodontal markers as risk indicators of early rheumatoid arthritis: a cross-sectional study, Clin Oral Investig 25 (2021) 1685–1695. P. Ruscitti, F. Ursini, P. Cipriani, M. Greco, S. Alvaro, L. Vasiliki, et al., IL-1 inhibition improves insulin resistance and adipokines in rheumatoid arthritis [155] [156] [157] [158] [159] [160] [161] [162] [163] [164] [165] [166] [167] [168] [169] [170] [171] [172] [173] [174] [175] [176] patients with comorbid type 2 diabetes: an observational study, Medicine 98 (2019) e14587. V. Chandran, F. Abji, A.V. Perruccio, R. Gandhi, S. Li, R.J. Cook, et al., Serumbased soluble markers differentiate psoriatic arthritis from osteoarthritis, Ann Rheum Dis 78 (2019) 796–801. V. Francisco, J. Pino, M.A. Gonzalez-Gay, A. Mera, F. Lago, R. Gómez, et al., Adipokines and inflammation: is it a question of weight?, Br J Pharmacol 175 (2018) 1569–1579 L. Liu, Z. He, L. Xu, L. Lu, H. Feng, D.J. Leong, et al., CITED2 mediates the mechanical loading–induced suppression of adipokines in the infrapatellar fat pad, Ann N Y Acad Sci 1442 (2019) 153–164. J. Martel-Pelletier, J.P. Raynauld, F. Mineau, et al., Levels of serum biomarkers from a two–year multicentre trial are associated with treatment response on knee osteoarthritis cartilage loss as assessed by magnetic resonance imaging: an exploratory study, Arthritis Res Ther 19 (2017). G. Valverde-Franco, G. Tardif, F. Mineau, F. Paré, B. Lussier, H. Fahmi, et al., High in vivo levels of adipsin lead to increased knee tissue degradation in osteoarthritis: data from humans and animal models, Rheumatology 57 (2018) 1851–1860. M.G.A. Ansari, S.D. Hussain, K.A. Wani, S.M. Yakout, D. Al-Disi, M.S. Alokail, et al., Influence of bone mineral density in circulating adipokines among postmenopausal Arab women, Saudi J Biol Sci 27 (2020) 374–379. B.F. O'Dowd, M. Heiber, A. Chan, H.H. Heng, L.C. Tsui, J.L. Kennedy, et al., A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11, Gene 136 (1993) 355–360. A.D. Medhurst, C.A. Jennings, M.J. Robbins, R.P. Davis, C. Ellis, K.Y. Winborn, et al., Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin, J Neurochem 84 (2003) 1162–1172. H. Antushevich, M. Wójcik, Review: apelin in disease, Clin Chim Acta 483 (May) (2018) 241–248. S. Liu, W. Wang, L. Yin, Y. Zhu, Influence of Apelin-13 on osteoporosis in Type2 diabetes mellitus: a clinical study, Pakistan J Med Sci 34 (2018) 159–163. T.K. Chang, Y.H. Wang, S.J. Kuo, S.W. Wang, C.H. Tsai, Y.C. Fong, et al., Apelin enhances IL-1b expression in human synovial fibroblasts by inhibiting miR-144-3p through the PI3K and ERK pathways, Aging (Albany NY) 12 (2020) 9224–9239. M. Di Franco, F.R. Spinelli, A. Metere, M.C. Gerardi, V. Conti, F. Boccalini, et al., Serum levels of asymmetric dimethylarginine and apelin as potential markers of vascular endothelial dysfunction in early rheumatoid arthritis, Mediators Inflamm 2012 (2012) 347268. C.C. Shu, C.R. Flannery, C.B. Little, J. Melrose, Catabolism of fibromodulin in developmental rudiment and pathologic articular cartilage demonstrates novel roles for MMP-13 and ADAMTS-4 in C-terminal processing of SLRPs, Int J Mol Sci 20 (2019) 1–21. T. Tahara, T. Shibata, M. Nakamura, H. Yamashita, D. Yoshioka, M. Okubo, et al., Effect of MDR1 gene promoter methylation in patients with ulcerative colitis, Int J Mol Med 23 (2009) 521–527. M. Blüher, C.S. Mantzoros, From leptin to other adipokines in health and disease: facts and expectations at the beginning of the 21st century, Metabolism 64 (2015) 131–145. K. Hida, J. Wada, H. Zhang, K. Hiragushi, Y. Tsuchiyama, K. Shikata, et al., Identification of genes specifically expressed in the accumulated visceral adipose tissue of OLETF rats, J Lipid Res 41 (2000) 1615–1622. C. Tu, J. He, B. Wu, W. Wang, Z. Li, An extensive review regarding the adipokines in the pathogenesis and progression of osteoarthritis, Cytokine 113 (May) (2019) 1–12. M. Ozgen, S.S. Koca, N. Dagli, M. Balin, B. Ustundag, A. Isik, Serum adiponectin and vaspin levels in rheumatoid arthritis, Arch Med Res 41 (2010) 457–463. L. Senolt, M. Polanská, M. Filková, L.A. Cerezo, K. Pavelka, S. Gay, et al., Vaspin and omentin: new adipokines differentially regulated at the site of inflammation in rheumatoid arthritis, Ann Rheum Dis 69 (2010) 1410–1411. N. Kamio, T. Kawato, N. Tanabe, S. Kitami, T. Morita, K. Ochiai, et al., Vaspin attenuates RANKL-induced osteoclast formation in RAW264.7 cells, Connect Tissue Res 54 (2013) 147–152. N. Tanna, K. Patel, A.E. Moore, D. Dulnoan, S. Edwards, G. Hampson, The relationship between circulating adiponectin, leptin and vaspin with bone mineral density (BMD), arterial calcification and stiffness: a cross-sectional study in post-menopausal women, J Endocrinol Invest 40 (2017) 1345–1353. T.P. Zhang, Y.L. Zhao, X.M. Li, C.H. Wu, H.F. Pan, D.Q. Ye, Altered mRNA expression levels of vaspin and adiponectin in peripheral blood mononuclear cells of systemic lupus erythematosus patients, Clin Exp Rheumatol 37 (2019) 458–464. www.drugdiscoverytoday.com 17 KEYNOTE Drug Discovery Today KEYNOTE KEYNOTE [177] V. Abella, J. Pino, M. Scotece, J. Conde, F. Lago, M.A. Gonzalez-Gay, et al., Progranulin as a biomarker and potential therapeutic agent, Drug Discov Today 22 (2017) 1557–1564. [178] J. Jian, G. Li, A. Hettinghouse, C. Liu, Progranulin: a key player in autoimmune diseases, Cytokine 101 (2018) 48–55. [179] C. Liu, X.X. Li, W. Gao, W. Liu, D.S. Liu, Progranulin-derived atsttrin directly binds to TNFRSF25 (DR3) and inhibits TNF-like ligand 1A (TL1A) activity, PLoS ONE 9 (2014) e92743. [180] W. Fu, W. Hu, L. Shi, J.J. Mundra, G. Xiao, M.L. Dustin, et al., Foxo4- and Stat3dependent IL-10 production by progranulin in regulatory T cells restrains inflammatory arthritis, FASEB J 31 (2017) 1354–1367. [181] J. Chen, S. Li, J. Shi, L. Zhang, J. Li, S. Chen, et al., Serum progranulin irrelated with Breg cell levels, but elevated in RA patients, reflecting high disease activity, Rheumatol Int 36 (2015) 359–364. [182] Y. Yamamoto, M. Takemura, G. Serrero, J. Hayashi, B. Yue, A. Tsuboi, et al., Increased serum GP88 (Progranulin) concentrations in rheumatoid arthritis, Inflammation 37 (2014) 1806–1813. [183] C. Kong, Y. Shi, J. Xu, Z. Xiu, W. Qi, Serum progranulin level is associated with disease activity following orthopedic surgery in rheumatoid arthritis patients, J Int Med Res 48 (2020). 300060520971459. [184] V. Abella, M. Scotece, J. Conde, V. López, C. Pirozzi, J. Pino, et al., The novel adipokine progranulin counteracts IL-1 and TLR4-driven inflammatory response in human and murine chondrocytes via TNFR1, Sci Rep 6 (2016) 20356. [185] J. Wei, K. Wang, A. Hettinghouse, C. Liu, Atsttrin promotes cartilage repair primarily through TNFR2-Akt pathway, Front Cell Dev Biol 8 (October) (2020) 1–10. [186] F. Qiu, L. Song, F. Ding, H. Liu, Q. Shu, N. Yang, et al., Expression level of the growth factor progranulin is related with development of systemic lupus erythematosus, Diagn Pathol 8 (2013) 1–7. [187] Y.J. Lan, N.B. Sam, M.H. Cheng, H.F. Pan, J. Gao, Progranulin as a potential therapeutic target in immune-mediated diseases, J Inflamm Res 14 (2021) 6543–6556. [188] W. Tang, Y. Lu, Q.Y. Tian, Y. Zhang, F.J. Guo, G.Y. Liu, et al., The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice, Science (80-) 332 (2011) 478–484. 18 www.drugdiscoverytoday.com Drug Discovery Today d Volume 27, Number 11 d November 2022 [189] H. Ding, J. Wei, Y. Zhao, Y. Liu, L. Liu, L. Cheng, Progranulin derived engineered protein Atsttrin suppresses TNF-a-mediated inflammation in intervertebral disc degenerative disease, Oncotarget 8 (2017) 109692–109702. [190] Q. Wang, Q. Xia, Y. Wu, X. Zhang, F. Wen, X. Chen, et al., 3D-Printed atsttrinincorporated alginate/hydroxyapatite scaffold promotes bone defect regeneration with TNF/TNFR signaling involvement, Adv Healthc Mater 4 (2015) 1701–1708. [191] S. Wang, G. Sun, P. Fan, L. Huang, Y. Chen, C. Chen, Distinctive roles of tumor necrosis factor receptor type 1 and type 2 in a mouse disc degeneration model, J Orthop Transl 31 (July) (2021) 62–72. [192] I. Ushach, G. Arrevillaga-Boni, G.N. Heller, et al., Meteorin-like/Meteorin-b is a novel immunoregulatory cytokine associated with inflammation, J Immunol 201 (2018) 3669–3676. [193] I. Ushach, A.M. Burkhardt, C. Martinez, et al., METEORIN-LIKE is a cytokine associated with barrier tissues and alternatively activated macrophages, Clin Immunol 156 (2015) 119–127. [194] C. Duerrschmid, Y. He, C. Wang, C. Li, J.C. Bournat, C. Romere, et al., Asprosin is a centrally acting orexigenic hormone, Nat Med 23 (2017) 1444–1453. [195] B. Basu, M. Jain, A.R. Chopra, Caudamins, a new subclass of protein hormones, Trends Endocrinol Metab 32 (2021) 1007–1014. [196] C. Romere, C. Duerrschmid, J. Bournat, P. Constable, M. Jain, F. Xia, et al., Asprosin, a fasting-induced glucogenic protein hormone, Cell 165 (2016) 566– 579. [197] Y.A.T. Morcos, S. Lütke, A. Tenbieg, F.G. Hanisch, G. Pryymachuk, N. Piekarek, et al., Sensitive asprosin detection in clinical samples reveals serum/saliva correlation and indicates cartilage as source for serum asprosin, Sci Rep 12 (2022) 1–19. [198] J. Heeren, L. Scheja, Isthmin 1 — a novel insulin-like adipokine, Nat Rev Endocrinol 17 (2021) 709–710. [199] Y. Wang, F. Sibaii, K. Lee, J.M. Gill, L.J. Hatch, Serum levels of the novel adipokine isthmin-1 are associated with obesity in pubertal boys, medRxiv 1 (2021) 1–13. [200] X. Zhao, Y. Dong, J. Zhang, D. Li, G. Hu, J. Yao, et al., Leptin changes differentiation fate and induces senescence in chondrogenic progenitor cells, Cell Death Dis 7 (2016) e2188.
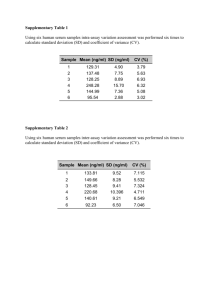
![Anti-Adiponectin antibody [1.3-13A05-2D09] ab131148](http://s2.studylib.net/store/data/013650560_1-ffde88290fcee38b48d6dd5d815267fb-300x300.png)