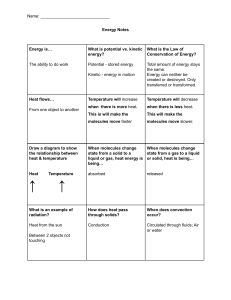
1. define heat and temperature 2. Differentiate between heat and temperature at the molecular level Looking Back The higher the frequency in the light wave means greater energy 2. The longer the wavelength the greater the energy 1. Red color has the least amount of energy 3. Looking Back 4. Violet is the color that is bent the most among the seven colors because it has the longest wavelength. 5. Refraction is the bending of light rays as they pass obliquely from one medium to another. Identify the following objects Sun What are these objects? Sources of Heat Candle Fire Kettle Flat iron Oven Lamp 5 6 Sources of Heat Sun Combustion Friction Electricity 7 is the transfer of thermal energy from one object to another because of temperature difference exists as “energy in transit” or in the process of being transferred and it is not contained in an object flow spontaneously from hot objects to 8 cold objects the energy that is actually contained in an object due to the motion of its particles. depends on the mass, temperature and phase ( solid, liquid or gas) of an object 9 Why do we need heat? dry our clothes keep us warm cooking 10 MEASURING HEAT Heat is measured by the units of calorie and joule (J). calorie: The amount of energy needed to raise the temperature of 1 gram of water by 1oC 1 calorie= 4.18 J the measure of the average kinetic energy of all the particles in the object due to their random motions through space It indicates the degree of hotness or coldness of a body or a place 12 Temperature is measured in units called degrees (oC, oF, oK) 13 14 16 Thermal expansion is where materials expand while being heated, causing them to take more space All gases, liquids, and most solids expand when their temperature increases. 18 19 20 What happens when molecules move more? More energy Molecules move more Molecules spread out More energy = EXPANSION 21 What happens when molecules move less? Less energy Molecules move less Molecules get closer together Less energy = CONTRACTION 22 What I Have Learned I have learned that________________ I have discovered that________________ I have realized that________________ 23 What I Have Learned I have learned that H_ _ _ _ is the transfer of T_ _ R_ _ L E _ e_ _y from one object to another I have discovered that heat flows from h_ _ body to c _ _ _ body I have realized that heat and temperature 24 are _____ the same Directions: Choose the letter of the correct answer 1. Heat transfers from an area of____temperature to an area of ___ temperature. A. Low to high B. Low to Low C. High to low D. High to high 25 Directions: Choose the letter of the correct answer 2. Temperature of a body measures the molecular_______. A. average kinetic energy B. average potential energy C. differences in kinetic energy D. differences in potential energy? 26 Directions: Choose the letter of the correct answer 3. Heat is the transfer of _____ from one object to another due to a difference in temperature. A. Current B. Energy C. Force D. Velocity 27 Directions: Choose the letter of the correct answer 4. What happens to the molecules of a substance as it gets cooler? A. B. C. D. The molecules expand The molecules move faster The molecules move slower The molecules shrink 28 Directions: Choose the letter of the correct answer 5. What is the unit of heat? A. Joule B. Kilogram C. Meter D. Newton 29 Answer 1.Heat transfers from an area of____temperature to an area of ___ temperature. C. High to low 2. Temperature of a body measures the molecular_______. A. average kinetic energy 3. Heat is the transfer of _____ from one object to another due to a difference in temperature B. Energy 4. What happens to the molecules of a substance as it gets cooler? C. The molecules move slower 5. What is the unit of heat? A. Joule 30 Looking forward 1. Explain the effects of heat on a body,( Phase Change) 2. Relate heat to mass and temperature through the concept of the specific heat capacity 31