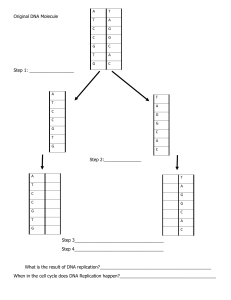
BCH323 Information flow and introduction to Bioinformatics Dr XH Makhoba LECTURE #2 – DNA Replication DNA dependent DNA polymerase DNA dependent RNA polymerase RNA dependent DNA polymerase RNA dependent RNA polymerase Ribosomes; mRNA; tRNA; rRNA Page 93 Voet Biochemistry 3e © 2004 John Wiley & Sons, Inc. Figure 5-22 Gene expression. Enzymes/Proteins required 1. 2. 3. 4. 5. 6. 7. 8. Proteins DnaA from dnaA gene and DnaC from the dnaC gene. DNA gyrase o Type II topoisomerase, introduces negative supercoils at the expense of ATP hydrolysis, to overcome torsional stress imposed by unwinding. Helicase (DnaB) o Synthesized from the dnaB gene o Separates the strands of DNA by hydrolyzing ATP and disrupts the hydrogen bonds holding the two strands together Single strand binding proteins o Prevent reannealing of the single stranded DNA Primase (DnaG) o 60 kD monomeric protein synthesized from the dnaG gene o DNA dependent RNA polymerase o Synthesizes a 1-60 bp long RNA primer on the lagging strand to initiate the synthesis of a Okazaki fragment (1000 – 2000 nt long in prokaryotes) o It is NOT inhibited by rifampicin (a RNA polymerase inhibitor) DNA dependent RNA polymerase o Together with Primase, mediates the initiation of the leading strand synthesis, which occurs only once in a replication cycle DNA dependent DNA polymerases o DNA polymerase I, II and III in E. coli o DNA polymerase , , and in Eukaryotes o Involved in de novo synthesis of DNA strands and DNA repair DNA ligase o Catalyzes the reaction that seals the single stranded nicks formed between adjacent Okazaki fragments, as well as those formed on circular DNA after leading strand synthesis DNA Replication Replication of duplex DNA is a complex mechanism that involves a whole group of enzyme activities involved in: 1) Initiation – recognition of an origin by a protein complex (primosome), which catalyze the separation of the parental strands, stabilize the single-stranded state and initiate synthesis of daughter strands at the replication fork 2) Elongation – protein complex (replisome) that is associated with the particular structure that DNA takes at the replication fork 3) Termination – at the end of replication joining and/or termination reactions are necessary followed by separation of the duplicate chromosomes from each other Prokaryotes • • • • • Initiation • Regulation of chromosomal replication occurs at the level of initiation. • Occurs at a unique genetic site, the replicative origin (ori). Enzymes/Proteins required include: • DNA gyrase • Helicase (DnaB) • Single strand binding proteins • Primase (DnaG) • DNA dependent RNA polymerase • Proteins DnaA (Recognizes and binds the DnaA boxes) and DnaC (an ATPase) The oriC is a unique 245-bp segment. The DnaA proteins recognize and bind the five DnaA boxes, each containing a highly conserved 9-bp consensus sequence (5’-TTATCCACA-3’), to form a complex of negatively supercoiled oriC DNA wrapped around a central core of five DnaA protein monomers. The DnaA proteins successively melt three tandemly repeated, 13-bp, AT-rich fragments (5’-GATCTNTTNTTTT-3’) located near the oriC. Two DnaB6-DnaC6 complexes are recruited to opposite ends of the replication “bubble” to form the PREPRIMING COMPLEX. Five additional DnaA proteins bind to the DnaA boxes to form five dimers at the DnaA boxes. The DnaC (an ATPase that facilitates DnaB loading) is subsequently released. Together with single strand binding proteins and gyrase, the DnaB helicase further unwinds the DNA in both directions allowing the entry of the primase and RNA polymerase Prokaryotes - Elongation Elongation • DNA Polymerases: • An enzyme that can synthesis a new DNA strand on a template strand. • Multiple DNA polymerase activities present – some involved with replication while others are involved in subsidiary roles or repair • All prokaryotic and Eukaryotic DNA polymerases share the same fundamental type of synthetic activity where the DNA strand is extended by adding nucleotides one at a time to a 3’-OH end dictated by base pairing with the template strand • DNA polymerases also have a mechanism to ensure fidelity of replication. This can be achieved by either scrutinizing the incoming base for proper complementarity with the template (presynthetic error control) OR scrutinize the base pair after the new base has been added (proofreading). All bacterial enzymes possess a 3’-5’ exonuclease activity that proceeds in the reverse direction from DNA synthesis, thus providing a proofreading control • Different DNA polymerases handle the relationship between the polymerizing and proofreading activities in different ways – sometimes it is part of the same protein subunit, sometimes it is contained in a different subunit Prokaryotes - Elongation • In E. coli three enzymes have been identified: • DNA polymerase I – involved in repair and in a subsidiary role in DNA semiconservative replication • DNA polymerase II – implicated in SOS response in DNA repair • DNA polymerase III – a multisubunit protein responsible for de novo synthesis of new DNA strands DNA Polymerase III • responsible for replication in E. coli. • It has several subunits: • the dnaE locus which codes for the 130kD subunit with DNA synthetic activity; • the dnaQ locus which codes for the 27.5 kD subunit with 3’-5’ exonuclease activity. It works in conjunction with the subunit by providing proofreading during DNA replication. • The role of the third 10kD subunit, , is unknown DNA polymerase errors include: • Substitutions due to the incorporation of a wrong nucleotide – this is corrected by the 3’-5’ proofreading activity of each DNA polymerase • Frameshifts when an extra nucleotide is inserted of left out – fidelity is affected by processivity (the tendency to remain on a single template rather than dissociate and reassociate). DNA polymerase I • A single polypeptide (103kD) • one subunit (68kD, Klenow fragment) containing polymerase and 3’-5’ exonuclease activity in different regions of the protein • one subunit (35kD) contains a 5’-3’ exonuclease activity which excises small groups of nucleotides up to ~10 bases at a time. • This activity is coordinated with the proofreading activity and enables DNA polymerase I to have the unique ability to start replication in vitro at a nick in DNA. This process is called nicked translation. The displaced strand is degraded by the 5’3’ exonuclease activity. • DNA Polymerase I in bacterium, is not responsible for replication. The 5’-3’ synthetic/3’-5’ exonucleolytic action is used: • In Nick translation, where the 5’-3’ activity removes the preceeding DNA at the nick and the polymerase activity replaces the nucleotides at the 3’-OH site. • In the excising of the RNA primers. Termination and segregation Termination of replication occurs at a specific site, diametrically opposite from oriC, named terC. • ter binding protein blocks the bidirectionally moving replicative forks from moving clockwise or counter clockwise. • The ter region contains a number of short DNA sequences containing a consensus core element 5’-GTGTGTTGT • A ter sequence element will empede replication fork progression only if orientated in the proper direction with respect to the approaching replication fork and only if a specific replication termination protein, Tus protein (a contahelicase), is bound to it. • Following termination of DNA replication, the resulting chromosomes must be segregated into daughter cells. DNA Polymerase • DNA is synthesized from its 5’ -> 3’ end (from the 3’ -> 5’ direction of the template) • the leading strand is synthesized continuously in the 5’ -> 3’ direction toward the replication fork • the lagging strand is synthesized semidiscontinuously (Okazaki fragments) also in the 5’ -> 3’ direction, but away from the replication fork • lagging strand fragments are joined by the enzyme DNA ligase DNA Polymerase Reaction • The 3’-OH group at the end of the growing DNA chain acts as a nucleophile. • The phosphorus adjacent to the sugar is attacked, and then added to the growing chain. Function of DNA Polymerase • DNA polymerase function has the following requirements: • all four deoxyribonucleoside triphosphates: dTTP, dATP, dGTP, and dCTP • Mg2+ • an RNA primer - a short strand of RNA to which the growing polynucleotide chain is covalently bonded in the early stages of replication • DNA-Pol I: repair and patching of DNA • DNA-Pol III: responsible for the polymerization of the newly formed DNA strand • DNA-Pol II, IV, and V: proofreading and repair enzymes Supercoiling and Replication • DNA gyrase (class II topoisomerase) catalyzes reaction involving relaxed circular DNA: • creates a nick in relaxed circular DNA • a slight unwinding at the point of the nick introduces supercoiling • the nick is resealed • The energy required for this process is supplied by the hydrolysis of ATP to ADP and Pi Replication with Supercoiled DNA • Replication of supercoiled circular DNA • DNA gyrase has different role here. It introduces a nick in supercoiled DNA • a swivel point is created at the site of the nick • the gyrase opens and reseals the swivel point in advance of the replication fork • the newly synthesized DNA automatically assumes the supercoiled form because it does not have the nick at the swivel point • helicase, a helix-destabilizing protein, promotes unwinding by binding at the replication fork • single-stranded binding (SSB) protein stabilizes single-stranded regions by binding tightly to them Primase Reaction • The primase reaction • RNA serves as a primer in DNA replication • primer activity first observed in-vivo. • Primase - catalyzes the copying of a short stretch of the DNA template strand to produce RNA primer sequence • Synthesis and linking of new DNA strands • begun by DNA polymerase III • the newly formed DNA is linked to the 3’-OH of the RNA primer • as the replication fork moves away, the RNA primer is removed by DNA polymerase I Replication of duplex DNA in E. coli. https://www.youtube.com/watch?v=-qAr5Ib_6as https://www.youtube.com/watch?v=FBYeBb4C5Rc https://www.khanacademy.org/science/high-school-biology/hs-molecular-genetics/hs-discovery-and-structure-of-dna/v/leading-and-lagging-strands-in-dna-replication Replication Fork General Features Replacement of RNA primers by DNA in lagging strand synthesis. Function of DNA ligase. The 3 5 exonuclease function of DNA polymerase I and DNA polymerase III. Summary of DNA Replication in Prokaryotes • DNA synthesis is bidirectional • DNA synthesis is in the 5’ -> 3’ direction • the leading strand is formed continuously • the lagging strand is formed as a series of Okazaki fragments which are later joined • Five DNA polymerases have been found to exist in E. coli • Pol I is involved in synthesis and repair • Pol II, IV, and V are for repair under unique conditions • Pol III is primarily responsible for new synthesis Summary of DNA Replication in Prokaryotes • Unwinding • DNA gyrase introduces a swivel point in advance of the replication fork • a helicase binds at the replication fork and promotes unwinding • single-stranded binding (SSB) protein protects exposed regions of singlestranded DNA • Primase catalyzes the synthesis of RNA primer • Synthesis • catalyzed by Pol III • primer removed by Pol I • DNA ligase seals remaining nicks Proofreading and Repair • DNA replication takes place only once each generation in each cell • Errors in replication (mutations) occur spontaneously only once in every 109 to 1010 base pairs • Can be lethal to organisms • Proofreading - the removal of incorrect nucleotides immediately after they are added to the growing DNA during replication • Errors in hydrogen bonding lead to errors in a growing DNA chain once in every 104 to 105 base pairs Proofreading Improves Replication Fidelity • Cut-and-patch catalyzed by Pol I: cutting is removal of the RNA primer and patching is incorporation of the required deoxynucleotides • Nick translation: Pol I removes RNA primer or DNA mistakes as it moves along the DNA and then fills in behind it with its polymerase activity • Mismatch repair: enzymes recognize that two bases are incorrectly paired, the area of mismatch is removed, and the area replicated again • Base excision repair: a damaged base is removed by DNA glycosylase leaving an AP site; the sugar and phosphate are removed along with several more bases, and then Pol I fills the gap The 5 3 exonuclease function of DNA polymerase I. DNA Polymerase Repair Mismatch Repair in Prokaryotes • Mechanisms of mismatch repair encompass: