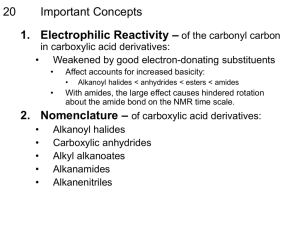
Study Guide Carboxylic Acids and Their Derivatives Chapters 20 and 21 1. Review of the Synthesis of Carboxylic Acids a. Oxidation of Alcohols CrO3/H2SO4 or K2Cr2O7 O or NaOCl OH OH b. Oxidative Cleavage of Alkenes O H O Big Dogs 3 H OH or KMnO4/H 2. New/Old Routes to Carboxylic Acids. Some of these routes you may have seen before and some will be new. a. Oxidation of Benzylic Carbons. Oxidation of benzylic carbons Possessing hydrogens. No Benzylic H’s O OH K2Cr2O7 or KMnO4 HO O Benzylic H’s b. Grignard with CO2. MgCl + O H 2O O C O OH 3. Nucleophilic Substitutions of Carboxylic Acid Derivatives. The fundamental reaction of carboxylic acid derivatives is the substitution of their leaving groups by nucleophiles. The Carboxylic Acid Energy Slide O Acid Chlorides Cl O O R O Cl Cl Oxalyl Chloride O Anhydrides R’ O O H 2O H 2O O S R Thioesters S H2O/H Cl Cl Thionyl Chloride O H2O/H R Esters O O OH O H2O/H R N H Amides Synthesis of Acid Chlorides. Carboxylic acids plus SOCl2 or C2O2Cl2. O O S + OH Cl Cl Thionyl Chloride O O O Cl O SO2 + HCl Gasses O + OH + Cl Cl Cl Oxalyl Chloride + CO2 + CO + HCl Gasses 4. Formation of Carboxylic Acid Derivatives. Starting at the Top. As discussed above, the acid chlorides are at the top of the carboxylic acid energy slide, and in principle, all of its derivatives can be made from them by use of the appropriate nucleophile. O O O R OH R O O SH S SOCl2 or O O C2O2Cl2 OH Cl OH O O NH2 O N H In turn, anhydrides can be converted to the lower derivatives by reaction with the appropriate nucleophiles. With anhydride electrophiles, the leaving group is a nice stable carboxylic acid. O O SH + S OH O O O O OH O + O OH O O NH2 + N OH H The lower three derivatives, esters, amides and carboxylic acids themselves can generally be converted to one another by using the appropriate nucleophile and driving the equilibrium in the desired direction by application of Le Chatelier’s principle, e.g., by use of high concentrations of nucleophile, distilling off the product or byproduct, or precipitation of the ammonium salt that would be formed with amines in acidic solution. O O H NH3 + + OH O N Precipitate Solvent H Fischer Esterification Reaction. By way of review, we learned the reaction of a carboxylic acid and an alcohol to make an ester and water back when we were studying alcohols. The mechanism of this reaction is a beautiful illustration of the five-step mantra in acidic solution. O O H + OH O OH Dean-Stark (-H2O) 1. Protonate 2. Attack by the nucleophile 3. Proton shuffle from the nucleophile to the wannabe leaving group 4. Reform the π-bond and dislodge the leaving group 5. Lose a proton Transesterification. A very similar reaction is the transesterification reaction that involves an ester with one alkoxide allowing to react with an alcohol to form an ester with a different alkoxide. The mechanism follows the 5-step mantra without missing a beat. O O H + + OH OH O O 5. Hydrolysis of Carboxylic Acid Derivatives. All of the carboxylic acid derivatives can be hydrolyzed to carboxylic acids under either acidic or basic conditions. The acid catalyzed and base assisted reactions both generally occur with their respective mechanisms for all the carboxylic acids. a. Acid Catalyzed Hydrolysis. The general mechanism is shown for the hydrolysis of an amide with an acid catalyst. Just to hammer this last point home, this next mechanism should look very familiar although I haven’t shown it to you before. O H OH N H N H HO – NH2 OH NH2 HO + H 2O OH OH NH OH2 -H O OH PS b. The Hydrolysis of Esters in Base. Carboxylic acid derivatives can also be hydrolyzed under aqueous basic conditions. There are times in a mechanism where you will need to add a proton, and under basic conditions, the source of a proton is water. Never use H+ in a basic solution because it does not exist. Saponification is the special term used for the hydrolysis of esters in base. This comes from soap making, which involves the basic hydrolysis of fatty esters to form fatty acids (soap) and glycerol. Note that this is a general hydrolysis that although demonstrated for esters, it holds for all of the carboxylic acid derivatives. Finally, the base is not a catalyst, it is a reagent that is consumed during the reaction. O O NaOH + OH OH O O O O O O + O O OH OH O OH Soap from Fatty Esters O O R1 O O O O R2 OH Very short lived because it’s deprotonated quickly O H2O/H Workup OH – + O NaOH HO R1, R2, R3 = Long chains, e.g., C18 OH + OH Glycerol R ONa Fatty Carboxylates (soap) R3 c. The Hydrolysis of Nitriles to Carboxylic Acids. Hidden among the carboxylic acid derivatives is the nitrile group. Of all of them it does not have a carbonyl, and therefore, doesn’t look like one. This nitrile disguise can be removed, however, in either an acid catalyzed or base assisted hydrolysis. c.1. Hydrolysis of Nitriles Under Acidic Conditions. Because the carboxylic acid product has two oxygens and the starting nitrile none, we will be adding a water nucleophile two times to the carbon of the nitrile. You will see a amide intermediate in this reaction. OH OH OH2 H PS H H 2O N N NH2 NH2 NH This is just a protonated amide OH OH OH O OH – NH3 PS -H NH + H 2O 2 NH3 NH2 OH OH2 OH OH c.2. Hydrolysis of Nitriles Under Basic Conditions. Use H2O as your proton source. Again, you will also see an amide is formed as an intermediate in this hydrolysis. N N + OH O NH2 O + NH2 OH OH NH3 OH – NH3 O This is just an amide O OH + OH Very short-lived because it deprotonates quickly O H2O/H Workup O O H 2O NH2 PS OH OH OH - OH O NH NH H 2O OH c.2.1 Making Nitriles by Dehydrating Amides. Noting above that amides are intermediates in the hydrolysis (adding water) of nitriles, it begs the question, can we dehydrate (remove water) amides to make nitriles? Yes, we can. Two good reagents for dehydrating amides are PCl3 and POCl3. The mechanism of dehydration is shown below. Remember phosphorous, with its 3d orbitals can act as a Lewis acid (accept a pair of electrons to form a bond), or act as a Lewis base using its 3s lone-pair electrons. In this case, PCl3 is acting as a Lewis acid and forming a bond to one of the two carbonyl lone-pairs. This is the same role it played in converting an alcohol to an alkyl chloride. O N NH2 O NH2 + Cl P Cl Cl PCl3/Et3N O - Cl PCl2 N H H + O NEt3 - HNEt3 PCl2 N H + NEt3 N - HNEt3 - OPCl2 5. The reactions of Carboxylic Acids with the Hard Organometallic Nucleophiles. Lithium, Grignard and Lithium Cuprates, all have metals with carbon bonds to them and these are referred to as organometallic compounds. These reactions should be familiar as we have seen the standard lithium and Grignard reagents reacting with carbonyl compounds of all flavors. 5.a. With Carboxylic Acids. Carboxylic acids with two equivalents of a lithium reagent will make a ketone. O O + 2 + Li H OH Ketone This is a two-step process with the first step being an acid-base reaction between the acid and the universe’s strongest base. O O + 1 Li + Li H O OH Hydrocarbon gas contributing to Global Warming The second step involves the expected attack on the carbonyl electrophile by the nucleophilic lithium reagent. The intermediate product is a dialkoxy dianion that is then protonated in the mildly acidic workup. Hydrates of course are unstable and will lose water to yield the final ketone. O O + 1 Li + Li H O OH O O Li + Li O O Li Li Dianion O O H2O/H Workup HO OH - H2O O Hydrate Dianion 5.b. Esters and Acid Chlorides. We were brought up on the reaction between esters and the hard organometallic reagents. The hard organometallic reagent will hit the carbonyl twice to yield 3° alcohols and a second alcohol in the case of the esters. O + Li Cl OH 3° Alcohols O + MgCl O 5.c.1. Softening up the Organometallics: Lithium Cuprates and Acid Chlorides. The hard lithium reagents can be softened up by forming lithium dialkyl cuprates, and these soft cuprates will react with 1° alkyl halides, and perform 1,4-addition to a,b-unsaturated carbonyls. We will now see that they will react with acid chlorides to do a single carbonyl hit, replacing the chloride leaving group, yielding a ketone. Br R O Li Cu (R2CuLi) SN2 R O 1,4-Addition O Cl O Cl Substitution or MgBr - 78°C One equivalent of Grignard reagent at very low temperature will also add one time to acid chlorides to yield the same ketone. 6. Reduction of Carboxylic Acid Derivatives. Again, many of these reactions should be familiar as we have seen LAH reducing carbonyl compounds of all flavors. We will also see some specialty hydride reagents have been developed to take the carboxylic acid derivatives to aldehydes rather than alcohols. 6.a. Complete Reductions. Carboxylic acid derivatives (acid chlorides, anhydrides, thioesters, acids and esters) can be reduced to 1° alcohols using LAH (this is very old school) and amines (amides). Note: Both carbonyls of the anhydride are reduced to alcohols, thioester reductions yield alcohols and thiols, and esters yield two alcohols. O H Cl O R1 H OH O O H R2 R1 O H S O LAH OH O H R2 + H OH SH H H OH H NH2 OH H OH H O + OH H O H + H NH2 OH 6.b. Reductions to Aldehydes. The reduction to aldehydes and not alcohols has been a more challenging chemical problem. There are new methods under development for the direct reduction of carboxylic acids to aldehydes. The two traditional two-step routes to aldehydes called on first: a) converting the acid to a 1° alcohol by reduction and then oxidizing it to the aldehyde using PCC, DMP, or the Swern oxidation; or 2) converting the acid to one of its derivatives and then reducing the derivative with one of the selective hydride reagents. OH LAH PCC or Swern O O ? OH H SOCl2 or C2O2Cl2 O LTBA Cl The specialty reducing agents we will use to reduce the carboxylic acid derivatives include DIBAL-H, LTBA and Rosenmund (Pd/BaSO4/H2). Except for acid chlorides, DIBAL-H and LTBA can be used interchangeably to yield the aldehyde. DIBAL-H is a little too strong and will reduce an acid chloride to the alcohol. H H Al Selective Hydride O O Li Al Carriers O Diisobutylaluminum Hydride Lithium Tris(t-Butoxy)Aluminum Hydride DIBAL-H LTBA 6.b.1 Summary of Acid Chloride Reductions. Acid chlorides, being the most reactive of the derivatives, have been the most difficult to reduce just to an aldehyde. A classic method is the Rosenmund reduction. Like Lindlar’s catalyst (a Pd/BaSO4/Quinoline/H2 to give cis-alkenes from triple bond reductions), Rosenmund’s catalyst is a poisoned concoction that can be used to reduce acid chlorides to aldehydes but it also promotes side reactions. A preferred reagent is LTBA. LAH H Alcohols H OH DIBAL–H O Cl Aldehydes Pd/BaSO4/H2 Rosenmund LTBA–H O H 6.b.2. Summary of Ester Reductions. Both DIBAL-H and LTBA will selectively reduce esters to aldehydes and the alcohol from the liberated alkoxide. H LAH Alcohols O OR OH DIBAL–H Aldehydes H + ROH O + ROH H LTBA–H 6.b.3. Summary of Amide Reductions. Amides are the most recalcitrant of the carboxylic acids, which is a good thing for us because we are largely held together by amide (peptide) bonds. Amides can be reduced to amines by LAH, or by the more selective regents to aldehydes. H H LAH Amines O NH2 NH2 DIBAL–H Aldehydes O H LTBA–H 6.b.4. Summary of Nitrile Reductions. Remember, nitriles are just carboxylic acids in disguise and they can be reduced to either the amines or the aldehydes. LAH H H Amines NH2 Ni/H2 N DIBAL–H O Aldehydes H LTBA–H 6.b.5. Summary of Thioester Reductions. Thioesters can be reduced with LAH to the thiol and alcohol. A selective reduction of thioesters to aldehydes was developed by Fukuyama using silanes (Si–H containing species) as the hydride source. LAH Thiols/Alcohols O S Pd/C, Et3Si–H Aldehydes H H + OH SH O H Fukuyama Reduction 7. A Little Something about Thioesters. Thioesters are used by biological organisms and one particular function they perform is adding an acyl group (a methyl group with a carbonyl, CH3CO, from acetic acid) to nucleophiles like alcohols. NH2 N O S H N OH H N O O O O O P O P O O O N N N O O OH O P O SCoA O The acetyl group is shown in blue and the large fuchsia segment is a thioester that acts as a leaving group. A great example of this is Acetyl Coenzyme A (Acetyl Co-A), which is involved in many functions and is used extensively in the citric acid (Krebs) cycle or in synthesis of Acetylcholine, an important neurotransmitter. O O OH SCoA N N + CoA–SH O Acetyl CoA OAc Acetylcholine Choline Acetate a Neurotransmitter Essential Nutrient O Acetyl Coenzyme A (Acetyl CoA) The Body’s Acid Chloride How utterly amazing is your body? Big time amazing in terms of being an organic synthesis machine. Here’s an example. Cholesterol shown below has 27 carbons, all of which come from a 2-carbon source: either the methyl carbon or the carbonyl carbon of acetyl CoA.