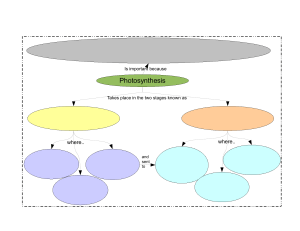
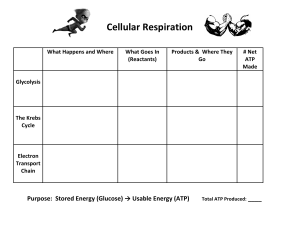
General Biology 1 Teaching Guide for Senior High School
advertisement

The Commission on Higher Education in collaboration with the Philippine Normal University Teaching Guide for Senior High School GENERAL BIOLOGY 1 SPECIALIZED SUBJECT | ACADEMIC-STEM This Teaching Guide was collaboratively developed and reviewed by educators from public and private schools, colleges, and universities. We encourage teachers and other education stakeholders to email their feedback, comments, and recommendations to the Commission on Higher Education, K to 12 Transition Program Management Unit - Senior High School Support Team at k12@ched.gov.ph. We value your feedback and recommendations. Development Team Team Leaders: Florencia G. Claveria, Ph.D., Dawn T. Crisologo Writers: Doreen D. Domingo, Ph.D., Aileen C. dela Cruz, Chuckie Fer A. Calsado, Doreen D. Domingo, Janet S. Estacion, Justin Ray M. Guce, Mary Jane C. Flores, Nolasco H. Sablan Technical Editors: Annalee S. Hadsall, Ph.D. Published by the Commission on Higher Education, 2016 Chairperson: Patricia B. Licuanan, Ph.D. Copy Reader: Caroline H. Pajaron Commission on Higher Education K to 12 Transition Program Management Unit Office Address: 4th Floor, Commission on Higher Education, C.P. Garcia Ave., Diliman, Quezon City Telefax: (02) 441-0927 / E-mail Address: k12@ched.gov.ph Cover Artists: Paolo Kurtis N. Tan, Renan U. Ortiz Illustrator: Ma. Daniella Louise F. Borrero Senior High School Support Team CHED K to 12 Transition Program Management Unit Program Director: Karol Mark R. Yee Consultants THIS PROJECT WAS DEVELOPED WITH THE PHILIPPINE NORMAL UNIVERSITY. University President: Ester B. Ogena, Ph.D. VP for Academics: Ma. Antoinette C. Montealegre, Ph.D. VP for University Relations & Advancement: Rosemarievic V. Diaz, Ph.D. Ma. Cynthia Rose B. Bautista, Ph.D., CHED Bienvenido F. Nebres, S.J., Ph.D., Ateneo de Manila University Carmela C. Oracion, Ph.D., Ateneo de Manila University Minella C. Alarcon, Ph.D., CHED Gareth Price, Sheffield Hallam University Stuart Bevins, Ph.D., Sheffield Hallam University Lead for Senior High School Support: Gerson M. Abesamis Lead for Policy Advocacy and Communications: Averill M. Pizarro Course Development Officers: John Carlo P. Fernando, Danie Son D. Gonzalvo Teacher Training Officers: Ma. Theresa C. Carlos, Mylene E. Dones Monitoring and Evaluation Officer: Robert Adrian N. Daulat Administrative Officers: Ma. Leana Paula B. Bato, Kevin Ross D. Nera, Allison A. Danao, Ayhen Loisse B. Dalena Printed in the Philippines by EC-TEC Commercial, No. 32 St. Louis Compound 7, Baesa, Quezon City, ectec_com@yahoo.com This Teaching Guide by the Commission on Higher Education is licensed under a Creative Commons AttributionNonCommercial-ShareAlike 4.0 International License. This means you are free to: Share — copy and redistribute the material in any medium or format Adapt — remix, transform, and build upon the material. The licensor, CHED, cannot revoke these freedoms as long as you follow the license terms. However, under the following terms: Attribution — You must give appropriate credit, provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use. NonCommercial — You may not use the material for commercial purposes. ShareAlike — If you remix, transform, or build upon the material, you must distribute your contributions under the same license as the original. Table of Contents Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 DepEd General Biology 1 Curriculum Guide . . . . . . . . . . . . . 5 Chapter 3: Energy Transformation Chapter 1: Cell Lesson 11: Photosynthesis and Cellular Respiration . . . . . . . . . . . 86 Lesson 1: The Cell: Endomembrane System, Mitochondria, 99 Chloroplasts, Cytoskeleton, and Extracellular Components . . . 9 Lesson 12: Forms of Energy, Laws of Energy Transformation and Role of ATP . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Lesson 2: Mitochondria and Chloroplasts . . . . . . . . . . . . . . . . . 15 Lesson 13: Energy Transformation Part 1 . . . . . . . . . . . . . . . . . . . . 111 Lesson 14: Energy Transformation Part 2 . . . . . . . . . . . . . . . . . . . . 120 Lesson 3: Structure and Functions of Animal Tissues and Cell Modification . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28 Lesson 15: Energy Transformation Part 3 . . . . . . . . . . . . . . . . . . . . 128 Lesson 4: Cell Cycle and Cell Division . . . . . . . . . . . . . . . . . . . . 36 Lesson 16: Cellular Respiration Part 1 . . . . . . . . . . . . . . . . . . . . . . 133 Lesson 5: Transport Mechanisms Part 1 . . . . . . . . . . . . . . . . . . . 46 Lesson 17: Cellular Respiration Part 2 . . . . . . . . . . . . . . . . . . . . . . 150 Lesson 6: Transport Mechanisms Part 2 . . . . . . . . . . . . . . . . . . . 50 Lesson 18: Cellular Respiration Part 3 . . . . . . . . . . . . . . . . . . . . . . 165 Chapter 2: Biological Molecules Lesson 19: ATP in Cellular Metabolism and Photosynthesis . . . . . 176 Lesson 7: Carbohydrates and Lipids . . . . . . . . . . . . . . . . . . . . . 57 Lesson 8: Amino Acids and Proteins Part 1 . . . . . . . . . . . . . . . . 70 Lesson 9: Amino Acids and Proteins Part 2 . . . . . . . . . . . . . . . . 73 Lesson 10: Biological Molecules: Enzymes . . . . . . . . . . . . . . . . 78 Biographical Notes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 184 Introduction As the Commission supports DepEd’s implementation of Senior High School (SHS), it upholds the vision and mission of the K to 12 program, stated in Section 2 of Republic Act 10533, or the Enhanced Basic Education Act of 2013, that “every graduate of basic education be an empowered individual, through a program rooted on...the competence to engage in work and be productive, the ability to coexist in fruitful harmony with local and global communities, the capability to engage in creative and critical thinking, and the capacity and willingness to transform others and oneself.” To accomplish this, the Commission partnered with the Philippine Normal University (PNU), the National Center for Teacher Education, to develop Teaching Guides for Courses of SHS. Together with PNU, this Teaching Guide was studied and reviewed by education and pedagogy experts, and was enhanced with appropriate methodologies and strategies. Furthermore, the Commission believes that teachers are the most important partners in attaining this goal. Incorporated in this Teaching Guide is a framework that will guide them in creating lessons and assessment tools, support them in facilitating activities and questions, and assist them towards deeper content areas and competencies. Thus, the introduction of the SHS for SHS Framework. SHS for SHS Framework The SHS for SHS Framework, which stands for “Saysay-Husay-Sarili for Senior High School,” is at the core of this book. The lessons, which combine high-quality content with flexible elements to accommodate diversity of teachers and environments, promote these three fundamental concepts: SAYSAY: MEANING HUSAY: MASTERY SARILI: OWNERSHIP Why is this important? How will I deeply understand this? What can I do with this? Through this Teaching Guide, teachers will be able to facilitate an understanding of the value of the lessons, for each learner to fully engage in the content on both the cognitive and affective levels. Given that developing mastery goes beyond memorization, teachers should also aim for deep understanding of the subject matter where they lead learners to analyze and synthesize knowledge. When teachers empower learners to take ownership of their learning, they develop independence and selfdirection, learning about both the subject matter and themselves. About this Teaching Guide Biology I is a Science, Technology, Engineering and Mathematics (STEM) Specialized Subject taken in the first half of Grades 11/12. Learners go on a journey geared toward the deeper understanding and appreciation of life processes at the cellular and molecular levels previously introduced in Grades 7-10. They will also apply basic chemistry and physics principles as they examine the transformation of energy in organisms. Implementing this course at the senior high school level is subject to numerous challenges with mastery of content among educators tapped to facilitate learning and a lack of resources to deliver the necessary content and develop skills and attitudes in the learners, being foremost among these. In support of the SHS for SHS framework developed by CHED, these teaching guides were crafted and refined by biologists and biology educators in partnership with educators from focus groups all over the Philippines to provide opportunities to develop the following: 1. Saysay through meaningful, updated, and context-specific content that highlights important points and common misconceptions so that learners can connect to their realworld experiences and future careers; 2. Husay through diverse learning experiences that can be implemented in a resourcepoor classroom or makeshift laboratory that tap cognitive, affective, and psychomotor domains are accompanied by field-tested teaching tips that aid in facilitating discovery and development of higher-order thinking skills; and 3. Sarili through flexible and relevant content and performance standards allow learners the freedom to innovate, make their own decisions, and initiate activities to fully develop their academic and personal potential. These ready-to-use guides are helpful to educators new to either the content or biologists new to the experience of teaching Senior High School due to their enriched content presented as lesson plans or guides. Veteran educators may also add ideas from these guides to their repertoire. The Biology Team hopes that this resource may aid in easing the transition of the different stakeholders into the new curriculum as we move towards the constant improvement of Philippine education. 2 Parts of the Teaching Guide This Teaching Guide is mapped and aligned to the DepEd SHS Curriculum, designed to be highly usable for teachers. It contains classroom activities and pedagogical notes, and is integrated with innovative pedagogies. All of these elements are presented in the following parts: 1. • • • • • 2. • • • • 3. • • • • 4. • • • • 5. • • • • 6. • • Introduction Highlight key concepts and identify the essential questions Show the big picture Connect and/or review prerequisite knowledge Clearly communicate learning competencies and objectives Motivate through applications and connections to real-life Motivation Give local examples and applications Engage in a game or movement activity Provide a hands-on/laboratory activity Connect to a real-life problem Instruction/Delivery Give a demonstration/lecture/simulation/hands-on activity Show step-by-step solutions to sample problems Give applications of the theory Connect to a real-life problem if applicable Practice Discuss worked-out examples Provide easy-medium-hard questions Give time for hands-on unguided classroom work and discovery Use formative assessment to give feedback Enrichment Provide additional examples and applications Introduce extensions or generalisations of concepts Engage in reflection questions Encourage analysis through higher order thinking prompts Evaluation Supply a diverse question bank for written work and exercises Provide alternative formats for student work: written homework, journal, portfolio, group/individual projects, student-directed research project On DepEd Functional Skills and CHED College Readiness Standards As Higher Education Institutions (HEIs) welcome the graduates of the Senior High School program, it is of paramount importance to align Functional Skills set by DepEd with the College Readiness Standards stated by CHED. On the other hand, the Commission declared the College Readiness Standards that consist of the combination of knowledge, skills, and reflective thinking necessary to participate and succeed without remediation - in entry-level undergraduate courses in college. The DepEd articulated a set of 21st century skills that should be embedded in the SHS curriculum across various subjects and tracks. These skills are desired outcomes that K to 12 graduates should possess in order to proceed to either higher education, employment, entrepreneurship, or middle-level skills development. The alignment of both standards, shown below, is also presented in this Teaching Guide - prepares Senior High School graduates to the revised college curriculum which will initially be implemented by AY 2018-2019. College Readiness Standards Foundational Skills DepEd Functional Skills Produce all forms of texts (written, oral, visual, digital) based on: 1. 2. 3. 4. 5. Solid grounding on Philippine experience and culture; An understanding of the self, community, and nation; Visual and information literacies, media literacy, critical thinking Application of critical and creative thinking and doing processes; and problem solving skills, creativity, initiative and self-direction Competency in formulating ideas/arguments logically, scientifically, and creatively; and Clear appreciation of one’s responsibility as a citizen of a multicultural Philippines and a diverse world; Systematically apply knowledge, understanding, theory, and skills for the development of the self, local, and global communities using prior learning, inquiry, and experimentation Global awareness, scientific and economic literacy, curiosity, critical thinking and problem solving skills, risk taking, flexibility and adaptability, initiative and self-direction Work comfortably with relevant technologies and develop adaptations and innovations for significant use in local and global communities Global awareness, media literacy, technological literacy, creativity, flexibility and adaptability, productivity and accountability Communicate with local and global communities with proficiency, orally, in writing, and through new technologies of communication Global awareness, multicultural literacy, collaboration and interpersonal skills, social and cross-cultural skills, leadership and responsibility Interact meaningfully in a social setting and contribute to the fulfilment of individual and shared goals, respecting the fundamental humanity of all persons and the diversity of groups and communities Media literacy, multicultural literacy, global awareness, collaboration and interpersonal skills, social and cross-cultural skills, leadership and responsibility, ethical, moral, and spiritual values 4 K to 12 BASIC EDUCATION CURRICULUM SENIOR HIGH SCHOOL – SCIENCE, TECHNOLOGY, ENGINEERING AND MATHEMATICS (STEM) SPECIALIZED SUBJECT Grade: Grade 11/12 Subject Title: Biology I Quarters: 1st to 2nd Quarter No. of Hours: 40 hours/10 Weeks per Quarter Subject Description: This subject is designed to enhance the understanding of the principles and concepts in the study of biology, particularly life processes at the cellular and molecular levels. It also covers the transformation of energy in organisms. Content Cell Content Standard The learners demonstrate an understanding of: 1. Cell Theory 2. Cell Structure and Functions 3. Prokaryotic vs Eukaryotic Cells 4. Cell Types 5. Cell Modifications Performance Standard The learners shall be able to: 1. construct a 3D model of a plant/animal/ bacterial cell using recyclable materials 2. construct a cell membrane model from indigenous or recyclable materials 6. Cell Cycle a. Mitosis b. Meiosis Learning Competencies The learners... 1. explain the postulates of the cell theory STEM_BIO11/12 -Ia-c-1 2. describe the structure and function of major and subcellular organelles STEM_BIO11/12 -Ia-c-2 3. distinguish prokaryotic and eukaryotic cells according to their distinguishing features STEM_BIO11/12 -Ia-c-3 4. classify different cell types (plant/animal tissues) and specify the function(s) of each STEM_BIO11/12 -Ia-c-4 5. describe some cell modifications that lead to adaptation to carry out specialized functions (e.g., microvilli, root hair) STEM_BIO11/12 -Ia-c-5 1. characterize the phases of the cell cycle and their control points STEM_BIO11/12 -Id-f-6 2. describe the stages of mitosis/meiosis given 2n=6 STEM_BIO11/12 -Id-f-7 STEM_BIO11/12 -Id-f-8 STEM_BIO11/12 -Id-f-9 3. discuss crossing over and recombination in meiosis 4. explain the significance or applications of mitosis/meiosis 7. Transport Mechanisms a. Simple Diffusion K to 12 Senior High School STEM Specialized Subject – General Biology 1 December 2013 Code 5. identify disorders and diseases that result from the malfunction of the cell during the cell cycle STEM_BIO11/12 -Id-f-10 1. describe the structural components of the cell STEM_BIO11/12 -Ig-h-11 Page 1 of 4 K to 12 BASIC EDUCATION CURRICULUM SENIOR HIGH SCHOOL – SCIENCE, TECHNOLOGY, ENGINEERING AND MATHEMATICS (STEM) SPECIALIZED SUBJECT Content Content Standard Performance Standard b. Facilitated Transport c. Active Transport d. Bulk/Vesicular Transport Biological Molecules Learning Competencies membrane Structures and Functions of Biological Molecules - Carbohydrates - Lipids - Proteins - Enzymes - Nucleic Acids 2. relate the structure and composition of the cell membrane to its function STEM_BIO11/12 -Ig-h-12 3. explain transport mechanisms in cells (diffusion osmosis, facilitated transport, active transport) STEM_BIO11/12 -Ig-h-13 4. differentiate exocytosis and endocytosis STEM_BIO11/12 -Ig-h-14 1. categorize the biological molecules(lipids, carbohydrates, proteins, and nucleic acids) according to their structure and function STEM_BIO11/12 -Ii-j-15 2. explain the role of each biological molecule in specific metabolic processes STEM_BIO11/12 -Ii-j-16 3. describe the components of an enzyme STEM_BIO11/12 -Ii-j-17 STEM_BIO11/12 -Ii-j-18 4. explain oxidation/reduction reactions Energy Transformation 1. ATP- ADP Cycle 2. Photosynthesis 3. Respiration Code prepare simple fermentation setup using common fruits to produce wine or vinegar via microorganisms K to 12 Senior High School STEM Specialized Subject – General Biology 1 December 2013 5. determine how factors such as pH, temperature, and substrate affect enzyme activity STEM_BIO11/12 -Ii-j-19 1. explain coupled reaction processes and describe the role of ATP in energy coupling and transfer STEM_BIO11/12 -IIa-j-1 2. describe the major features and chemical events in photosynthesis and respiration STEM_BIO11/12 -IIa-j-2 3. explain the importance of chlorophyll and other pigments STEM_BIO11/12 -IIa-j-3 4. describe the patterns of electron flow through light reaction events STEM_BIO11/12 -IIa-j-4 5. describe the significant events of the Calvin cycle STEM_BIO11/12 -IIa-j-5 Page 2 of 4 K to 12 BASIC EDUCATION CURRICULUM SENIOR HIGH SCHOOL – SCIENCE, TECHNOLOGY, ENGINEERING AND MATHEMATICS (STEM) SPECIALIZED SUBJECT Content Content Standard Performance Standard K to 12 Senior High School STEM Specialized Subject – General Biology 1 December 2013 Learning Competencies Code 6. differentiate aerobic from anaerobic respiration STEM_BIO11/12 -IIa-j-6 7. explain the major features and sequence the chemical events of cellular respiration STEM_BIO11/12 -IIa-j-7 8. distinguish major features of glycolysis, Krebs cycle, electron transport system, and chemiosmosis STEM_BIO11/12 -IIa-j-8 9. describe reactions that produce and consume ATP STEM_BIO11/12 -IIa-j-9 10. describe the role of oxygen in respiration and describe pathways of electron flow in the absence of oxygen STEM_BIO11/12 -IIa-j-10 11. compute the number of ATPs needed or gained in photosynthesis and respiration STEM_BIO11/12 -IIa-j-11 12. explain the advantages and disadvantages of fermentation and aerobic respiration STEM_BIO11/12 -IIa-j-12 Page 3 of 4 K to 12 BASIC EDUCATION CURRICULUM SENIOR HIGH SCHOOL – SCIENCE, TECHNOLOGY, ENGINEERING AND MATHEMATICS (STEM) SPECIALIZED SUBJECT Code Book Legend Sample: STEM_BIO11/12-IIa-j-12 LEGEND SAMPLE Learning Area and Strand/ Subject or Specialization Science, Technology, Engineering and Mathematics Grade Level Grade 11 or 12 Domain/Content/ Component/ Topic General Biology First Entry Uppercase Letter/s STEM_BIO11/12 Roman Numeral *Zero if no specific quarter Lowercase Letter/s *Put a hyphen (-) in between letters to indicate more than a specific week Quarter Second Quarter II Week Weeks one to ten a-j - Arabic Number Competency K to 12 Senior High School STEM Specialized Subject – General Biology 1 December 2013 explain the advantages and disadvantages of fermentation and aerobic respiration 12 Page 4 of 4 General Biology 1 60 MINS The Cell: Endomembrane System, Mitochondria, Chloroplasts, Cytoskeleton, and Extracellular Components Content Standards The learners demonstrate an understanding of (1) Composition of the endomembrane system; (2) Structure and function of organelles involved in energy transformation; (3) Structure and functions of the cytoskeleton; and, (4) Composition and functions of the extracellular components or matrix. Performance Standards The learners shall be able to construct three-dimensional models of whole cells using indigenous or recyclable materials. The models shall show the following cell parts: (1) Endomembrane System, (2) Mitochondria, and (3) Chloroplast Learning Competencies The learners: (1) explain the postulates of the cell theory (STEM_BIO11/12-1ac-1); (2) describe the structure and function of major and subcellular organelles (STEM_BIO11/12-Ia-c-2); (3) describe the structural components of the cell membrane (STEM_BIO11/12-Ig-h-11); and (4) relate the structure and composition of the cell membrane to its function (STEM_BIO11/12-Ig-h-12) Specific Learning Outcomes At the end of the unit lesson, the learners shall be able to: • • • • • illustrate the structure of the endomembrane system, label its parts, and understand how the system works illustrate the structure of the mitochondria, label its parts, and understand the importance of the enfolding of the inner mitochondrial membrane illustrate the structure of the chloroplast, label its parts, and relate these parts to photosynthesis understand the connection of the endomembrane system to other cell parts such as the lysosomes, peroxisomes, endosomes, and cell membrane understand how the extracellular components or matrix determine the appearance and function of the tissues LESSON OUTLINE Introduction Review on the differences between 5 prokaryotic and eukaryotic cells; submission and discussion of responses to the pre-topic homework assigned before the lecture. Motivation 5 Brief class activity on prokaryotic and eukaryotic cells. Instruction/ Lecture. Board work on cell parts, structure, Practice and function. Examination of cheek cells and 40 Hydrilla cells under a microscope. Class activity on identifying the parts and functions of the endomembrane system. Enrichment Class discussion on cell size and relationship of surface area and volume Evaluation Assessment of learners’ knowledge; assignment of homework for next lecture Materials microscope (slide, cover slip), hand-held lens, work books, methylene blue, plastic spoon/popsicle stick, Hydrilla plansts, colored chalk/white board marker 5 5 Resources (continued at the end of Teaching Guide) (1) (n.d.). Retrieved from <http://www.phschool.com/science/ biology_place/biocoach/cells/common.html> (2) (n.d.). Retrieved from <http://biology.tutorvista.com/animal-and-plant(3) (n.d.). Retrieved from <http://sciencenetlinks.com/lessons/cells-2-thecell-as-a-system/> INTRODUCTION (5 MINS) Teacher Tip 1. Ask the learners to make a recap of the differences between prokaryotic and eukaryotic cells. 2. Discuss the learners’ responses to the pre-topic assignment on the functions of the following cell parts: • Nucleus The review on the differences between prokaryotic and eukaryotic cells is needed to connect prerequisite knowledge to the present lesson. Remind the learners that the cell parts are found in eukaryotic cells. Remind the learners of the pre-topic assignment that shall be submitted before the lecture. This is to ensure the learners read on the topic before the lecture. • Smooth Endoplasmic Reticulum • Rough Endoplasmic Reticulum • Golgi Apparatus • Ribosomes Briefly discuss the structure of the cell membrane in order to provide basic knowledge on said structure to the learners. Do not fully elaborate on this topic since the structure and function of the cell membrane shall further be discussed in the succeeding parts of the lesson. • Lysosomes • Mitochondria • Chloroplast 3. Present an overview of the cell membrane, its structure, and functions. 4. Define what an ‘organelle’ is and differentiate membrane-bound organelles from non-membranebound organelles. 5. Explain that in eukaryotic cells, the machinery of the cell is compartmentalized into organelles. The compartmentalization of the cell into membrane-bound organelles: • allows conflicting functions (i.e., synthesis vs. breakdown) and several cellular activities to occur simultaneously without interference from each other • separates the DNA material of the nucleus, mitochondria, and chloroplast • increases the surface area-volume ratio of the cell 6. Encourage the learners to look at the cell as both a system and subsystem. They should develop an understanding of how the parts of a cell interact with one another and how these parts help to do the ‘work’ of the cell (Source: (n.d.). Retrieved from <http://sciencenetlinks.com/lessons/cells-2-the-cell-asa-system/>) 10 The cell’s parts should be discussed as a system, emphasizing on the interconnectedness of each part to the others. To clarify common misconceptions, emphasize the following to the learners: • Not all organelles are surrounded by a membrane. • The plasma or cell membrane is different from the cell wall. • Not all cell parts are present in all kinds of cells. MOTIVATION (5 MINS) Briefly review the differences between prokaryotic and eukaryotic cells by asking questions to the learners. Sample question: What cell parts can be found in both prokaryotic and eukaryotic cells? Discuss the function/s of each part. Sample Responses: • DNA • Cell membrane • Protoplasm (nucleoloid region and cytosol) • Ribosomes Compare the cell to a big city. Ask the learners what the requirements of the city would be in order for it to function. Relate these requirements to the parts of the cell. Relate the learners’ responses to the functions of the different parts of a cell. Sample responses: • The city will need power. What generates power for the city? Relate this to the function of the mitochondria and the chloroplast. • The city generates waste. How does it minimize its waste? How does the city handle its garbage? Relate this to the function of the lysosome. • The city requires raw materials to process into food, clothing, and housing materials. Where are these raw materials processed? Relate this to the functions of the Golgi Apparatus. Compare animal cells from plant cells. For the animal cells, scrape cheek cells using a toothpick. Ask the learners to place the scrapings on a microscope slide and add a drop of water to the scrapings. Tease the scrapings into a thin layer and cover with a slip. Examine under HPO. Instruct the learners to draw the cells on their workbooks and to label the cell parts that they were able to observe under the microscope. For the plant cells, instruct the learners to obtain a Hydrilla leaf and place it on a microscope slide. Examine under LPO. Ask the learners to draw the cells on their workbooks and to label the cell parts that they were able to observe under the microscope. Teacher tip If the number of available microscopes is limited, ask the learners to group themselves according to the number of microscopes available or set-up a demonstration scope for the whole class and facilitate the examination of cells so that all the learners will get a chance to observe the cells under the microscope. Orient the learners on the proper use and care of the microscopes, particularly on focusing first on LPO before shifting to HPO. Cheek cells are very transparent. Adjust the iris diaphragm or add a small amount of dye (i.e., methylene blue) to the scrapings. The learners will only see the cell membrane and the nucleus. Remind the learners to draw what they observe. Students may observe cytoplasmic streaming in the plant cell. INSTRUCTION/PRACTICE (30 MINS) 1. Draw the cell membrane on one end of the board. 2. Draw the double membrane of the nucleus (nuclear membrane) on the other end of the board. 3. From the nuclear membrane, draw the reticulated structure of the endoplasmic reticulum. Ask the learners what the two types of endoplasmic reticulum are and their corresponding functions. 4. Draw the ribosomes as separate units. 5. Draw a DNA and an mRNA. Explain that the mRNA is a copy of the DNA that will be sent to the cytoplasm for protein synthesis. 6. Explain to the learners that the mRNA leaves the nucleus and goes to where the ribosomes are located (i.e., mRNA + functional ribosome) 7. Explain the possible ‘pathways’ for protein synthesis (e.g., within the cytosol or the endoplasmic reticulum) 8. Draw the mRNA + functional ribosome on the endoplasmic reticulum. With a lot of these, the endoplasmic reticulum becomes a rough endoplasmic reticulum. 9. Draw the formed polypeptide inside the rough endoplasmic reticulum. Discuss the formation of a cisternae and pinching off as a vesicle. 10. Draw the Golgi Apparatus and then a vesicle from the rough endoplasmic reticulum that travels to the Golgi Apparatus and attaches to the part which is nearest the rough endoplasmic reticulum. 11. Ask the learners what the function of the Golgi Apparatus is. Synthesize their answers and compare the Golgi Apparatus to a factory with an assembly manufacturing line. 12. Draw the polypeptide travelling along the Golgi Apparatus stack; pinching off as a vesicle to travel to the next stack. Repeat the process while increasing the complexity of the polypeptide drawing. 13. On the last stack, explain the ‘pathways’ that the vesicle may follow: become a lysosome through fusion with an endosome (i.e., formed by endocytosis), or travel to the cell membrane, fuse with it, and empty its contents. 14. Present the composition of the endomembrane system and discuss how these parts are connected to each other by structure and by function. 15. Draw the mitochondria and label its parts. Explain the importance of the enfolding (cristae) in increasing the surface area of the inner mitochondrial membrane. Further explain to the class that Teacher tip Use chalk or white board markers with different colors. Explain the structure and function of each cell part as you draw them. Explain to the learners that a more detailed discussion of the structure and functions of the cell membrane, mitochondria, and chloroplast will be given in succeeding lessons. enfolding is a common structural strategy to increase surface area. As an example, you may draw a cross-sectional structure of the small intestine. 16. Draw the chloroplast and label its parts. Explain the function that each part performs in the process of photosynthesis. 17. Discuss the similarities of the mitochondria and chloroplast (e.g., both are involved in energy transformation, both have DNA, high surface area, and double membranes).income accounts and lastly, expenses accounts. Group the learners into pairs. Ask one to draw the endomembrane system as he/she explains it to his/her partner. Reshuffle the groupings and repeat until all learners have performed the exercise. ENRICHMENT (30 MINS) Facilitate a class discussion on why cells are generally small in size. Explain the relationship between surface area and volume. EVALUATION (60 MINS Ask questions to the learners. Sample questions can be found in the following electronic resources: Teacher tip • (n.d.). Retrieved from< http://www.proprofs.com/quiz-school/story.php?title=cell-structure-test > • (n.d.). Retrieved from< http://study.com/academy/exam/topic/cell-biology.html> Assign a research assignment on this question: How do environmental toxins like lead and mercury affect the functions of the cell? The assignment shall be submitted one week after this lesson. Assignments should be handwritten. RESOURCES (CONTINUED): (4) (n.d.). Retrieved from <http://www.schools.manatee.k12.fl.us/072JOCONNOR/celllessonplans/ lesson_plan__cell_structure_and_function.html> (5) (n.d.). Retrieved from <http://www.phschool.com/science/biology_place/biocoach/cells/endo.html> (6) (n.d.). Retrieved from <http://study.com/academy/lesson/the-endomembrane-system-functionscomponents.html> (7) (n.d.). Retrieved from <http://www.ncbi.nlm.nih.gov/books/NBK26907/> (8) (n.d.). Retrieved from <http://staff.um.edu.mt/acus1/01Compart.pdf> This strategy is aimed at ensuring that the learners have read the topic rather than just copying and printing from a source. ASSESSMENT Learning Competency Assessment Tool Exemplary Learner was able to Learner participation (during answer all the question/s without referring to his/ 1. describe the structure and lecture) her notes function of major and The learners shall be able to: subcellular organelles (STEM_BIO11/12-Ia-c-2) The learners shall be able to: 2. describe the structural components of the cell membrane (STEM_BIO11/12-Ig-h-11) The learners shall be able to: 3. relate the structure and composition of the cell membrane to its function (STEM_BIO11/12-Ig-h12) Assignment Learner submitted an assignment beyond the requirements Learner was able to Learner participation (during concisely answer all the questions practice) Satisfactory Developing Learner was able to answer the main question without referring to his/her notes but was not able to answer follow-up question/s Learner was able to answer the questions but he/she referred to his/her notes (1) Learner was not able to answer the question/s Learner submitted a comprehensive and wellwritten assignment Learner submitted a well written report but some responses lack details (1) Learner did not submit an assignment Learner was able to answer the main question without referring to his/her notes but was not able to answer follow-up question/s Learner was able to answer the questions but he/she referred to his/her notes Learner submitted drawings that fulfilled the requirements (complete and detailed) Learner submitted drawings that were incomplete (1) Learner was not able to submit drawings (2) Learner’s drawings were haphazardly done Learner obtained less that 50% correct answers in the examination Laboratory (Examination of Animal and Plant Cells) Learner submitted drawings that were beyond the requirements Examination Learner obtained 90% to 100% correct answers in the examination Learner obtained 70% to 89.99% correct answers in the examination Learner obtained 50% to 69.99% correct answers in the examination Research Assignment Learner submitted a research assignment beyond the requirements Learner submitted a comprehensive and wellwritten research assignment Learner submitted a well written report but some responses lack details 14 Beginnning (2) Learner read notes of his/her classmate (2) Learner submitted a partially-finished assignment (1) Learner was not able to answer the question/s (2) Learner read notes of his/her classmate (1) Learner did not submit an assignment (2) Learner submitted a partially-finished assignment General Biology 1 60 MINS Mitochondria and Chloroplasts Content Standards LESSON OUTLINE The learners demonstrate an understanding of the structure and function of the mitochondria and chloroplasts, the organelles involved in energy Introduction Review of relevant terminologies and definitions transformation. 5 Performance Standards The learners shall be able to construct three-dimensional models of whole cells using indigenous or recyclable materials. These models should show the mitochondria and chloroplasts. Motivation Instruction/ Discussion and lecture proper Delivery 30 Learning Competencies The learners describe the structure and function of major and subcellular organelles (STEM_BIO11/12-Ia-c-2) and distinguish prokaryotic and eukaryotic cells according to their distinguishing features (STEM_BIO11/12 -Ia-c-3) Practice 10 5 Understanding of key concepts using real-life situations Drawing (with label) activity Enrichment Computation of surface area vs volume 5 Evaluation 5 Answering practice questions and homework Resources (continued at the end of Teaching Guide) Specific Learning Outcomes At the end of the lesson, the learners shall be able to: • • illustrate the structure of the mitochondria, label its parts, and understand the importance of the enfolding of the inner mitochondrial membrane illustrate the structure of the chloroplast, label its parts, and relate these parts to photosynthesis (1) http://scienceaid.co.uk/biology/biochemistry/atp.html (2) http://www.britannica.com/list/6-cell-organelles) (3) http://www.nature.com/scitable/topicpage/mitochondria-14053590) (4) http://www.britannica.com/list/6-cell-organelles (5) http://www.nature.com/scitable/topicpage/mitochondria-14053590) (6) http://biology.tutorvista.com/animal-and-plant-cells/chloroplasts.html (7) ttp://www.nature.com/scitable/topicpage/mitochondria-14053590 INTRODUCTION (5 MINS) Facilitate a review of the following concepts: • • • • • Differences between prokaryotic and eukaryotic cells Definition of an ‘organelle’ Differences between membrane-bound organelles and non-membrane-bound organelles Functions of the different parts of a cell The endomembrane system MEMBRANE-BOUND ORGANELLES NON-MEMBRANE-BOUND ORGANELLES Nucleus Ribosomes Smooth ER Centrioles Rough ER Cytoskeleton Golgi Apparatus Vacuoles and Vesicles Mitochondria Chloroplast and other plastids Lysosomes Peroxisomes Explain that in eukaryotic cells, the machinery of the cell is compartmentalized into organelles. The compartmentalization of the cell into membrane-bound organelles: • • • allows conflicting functions (i.e., synthesis vs. breakdown) and several cellular activities to occur simultaneously without interference from each other separates the DNA material of the nucleus, mitochondria, and chloroplast increases the surface area-volume ratio of the cell 16 Encourage the learners to look at the cell as both a system and subsystem. They should develop an understanding of how the parts of a cell interact with one another and how these parts help to do the ‘work’ of the cell (Source: (n.d.). Retrieved from <http://sciencenetlinks.com/lessons/cells-2-the-cell-asa-system/>) Emphasize to the learners that energy transformation is one of the characteristics of life. This refers to the ability to obtain and use energy. This characterizes the main function of the mitochondria and the chloroplasts. MOTIVATION (5 MINS) Ask the learners how they understand the concept of compartmentalization. Relate the concept to how the cell is compartmentalized into organelles. Compare compartmentalization to the division of a house into a receiving room or sala, kitchen, dining room, comfort rooms, bedrooms, etc. Teacher tip Ask the learners why they think a house is divided into several rooms. A possible response is that partitioning of the house into different parts facilitates the simultaneous occurrence of several activities without interfering with one another. Also, materials needed for each activity can be stored at their specific areas. For example, pots and pans are being stored in the kitchen and not in the bedroom. Beds and pillows are found in the bedroom and not in the toilet/bath. Explain to the learners that the mitochondria and chloroplasts have a small amount of DNA. Although most of the proteins of these organelles are imported from the cytosol and are thus programmed by the nuclear DNA, their DNA programs the synthesis of the proteins made on the organelles’ ribosomes (Source: Campbell et al). Compartmentalization separates the DNA material of the nucleus, mitochondria, and chloroplast. Ask the learners if they have experienced going to a city/municipal hall and if they have observed that the Mayor, Vice-Mayor, and the City/Municipal Administrator have separate offices. You can use other examples such as the University President, VP for Academic Affairs, VP for Finance; Philippine President, Vice President, Senators, etc. Compare the nuclear DNA to the Mayor and the mitochondrial DNA and chloroplast DNA to the Vice Explain to the learner that this is how the cell is able to allow conflicting functions (e.g., synthesis vs breakdown) and several cellular activities to occur simultaneously without interference from each other. Mayor. The Mayor runs the city/municipality but the Vice Mayor also performs functions that are specific to their positions. They need different offices (or compartments) to avoid conflict in their functions. Teacher tip Select a fruit that can be easily peeled like calamansi or dalandan Introduce the concept of surface area-volume ratio/relationship to the learners. Show a fruit to the learners and explain that the outer surface of the fruit is the surface area. Peel the fruit and show them what’s inside, explaining that the inside of the fruit is the volume. Explain to the learners that surface area (SA) and volume (V) do not increase in the same manner. As an object increases in size, its volume increases as the cube of its linear dimensions while surface area increases as the square of its linear dimensions. Example: If the initial starting point is the same: SA = 2; Volume = 2 (Ratio = 1:1) A one-step increase will result to: SA = 22 = 4 while V = 23 = 8 (Ratio = 1:2) Teacher tip Ask questions to the learners while giving the lecture. INSTRUCTION/DELIVERY (30 MINS) Explain and discuss the nature and functions of the Adenosine Triphosphate (ATP) to the learners. Adenosine Triphosphate (ATP)—It is the major energy currency of the cell that provides the energy for most of the energy-consuming activities of the cell. The ATP regulates many biochemical pathways. Mechanism: When the third phosphate group of ATP is removed by hydrolysis, a substantial amount of free energy is released. ATP + H2O → ADP + Pi where ADP is adenosine diphosphate and Pi is inorganic phosphate Group the learners into pairs. Ask one to draw the endomembrane system as he/she explains it to his/ her partner. Reshuffle the groupings and repeat until all learners have performed the exercise. 18 If an LCD projector is not available, draw the structure of the mitochondria and chloroplast on the board. Illustration 1: Energy release in Hydrolysis (Source: (n.d.). Retrieved from http://scienceaid.co.uk/biology/biochemistry/atp.html) Illustration 2: Chemical Energy and ATP (Source: (n.d.). Retrieved from http://winklebiology.weebly.com/chemical-energyatp.html) Synthesis of ATP • ADP + Pi → ATP + H2O • requires energy: 7.3 kcal/mole • occurs in the cytosol by glycolysis • • occurs in mitochondria by cellular respiration occurs in chloroplasts by photosynthesis Consumption of ATP ATP powers most energy-consuming activities of cells, such as: • • • • • • • • anabolic (synthesis) reactions, such as: joining transfer RNAs to amino acids for assembly into proteins synthesis of nucleoside triphosphates for assembly into DNA and RNA synthesis of polysaccharides synthesis of fats active transport of molecules and ions conduction of nerve impulses maintenance of cell volume by osmosis • addition of phosphate groups (phosphorylation) to different proteins (e.g., to alter their activity in cell signaling) • • • muscle contraction beating of cilia and flagella (including sperm) bioluminescence Extracellular ATP In mammals, ATP also functions outside of cells. ATP is released in the following examples: • • • • from damaged cells to elicit inflammation and pain from the carotid body to signal a shortage of oxygen in the blood from taste receptor cells to trigger action potentials in the sensory nerves leading back to the brain from the stretched wall of the urinary bladder to signal when the bladder needs emptying In eukaryotic cells, the mitochondria and chloroplasts are the organelles that convert energy to other forms which cells can use for their functions. Discuss the function and structure of the mitochondria. 20 Mitochondria (singular, mitochondrion)—Mitochondria are the sites of cellular respiration, the metabolic process that uses oxygen to drive the generation of ATP by extracting energy from sugars, fats, and other fuels. The mitochondria are oval-shaped organelles found in most eukaryotic cells. They are considered to be the ‘powerhouses’ of the cell. As the site of cellular respiration, mitochondria serve to transform molecules such as glucose into an energy molecule known as adenosine triphosphate (ATP). ATP fuels cellular processes by breaking its high-energy chemical bonds. Mitochondria are most plentiful in cells that require significant amounts of energy to function, such as liver and muscle cells. Figure 1: Structure of the Mitochonsdria (Source: (n.d.). Retrieved from http://www.britannica.com/list/ 6-cell-organelles) The mitochondria has two membranes that are similar in composition to the cell membrane: • • Outer membrane—is a selectively permeable membrane that surrounds the mitochondria. It is the site of attachment for the respiratory assembly of the electron transport chain and ATP Synthase. It has integral proteins and pores for transporting molecules just like the cell membrane Inner membrane—folds inward (called cristae) to increase surfaces for cellular metabolism. It contains ribosomes and the DNA of the mitochondria. The inner membrane creates two enclosed spaces within the mitochondria: • intermembrane space between the outer membrane and the inner membrane; and • matrix that is enclosed within the inner membrane. Ask questions to the learners on the structure of the mitochondria. A sample question could be: What is the importance of the enfolding of the mitochondria? The response would be to increase the surface area that can be ‘packed’ into such a small space. Discuss the purpose of the mitochondrial membranes. 22 As mentioned, the mitochondria has two membranes: the outer and inner mitochondrial membranes. • • Outer Membrane • fully surrounds the inner membrane, with a small intermembrane space in between • has many protein-based pores that are big enough to allow the passage of ions and molecules as large as a small protein Inner membrane • has restricted permeability like the plasma membrane • is loaded with proteins involved in electron transport and ATP synthesis • surrounds the mitochondrial matrix, where the citric acid cycle produces the electrons that travel from one protein complex to the next in the inner membrane. At the end of this electron transport chain, the final electron acceptor is oxygen, and this ultimately forms water (H20). At the same time, the electron transport chain produces ATP in a process called oxidative phosphorylation During electron transport, the participating protein complexes push protons from the matrix out to the intermembrane space. This creates a concentration gradient of protons that another protein complex, called ATP synthase, uses to power synthesis of the energy carrier molecule ATP. Figure 4: The Electrochemical Proton Gradient and the ATP Synthase (Source: (n.d.). Retrieved from http://www.nature.com/scitable/topicpage/mitochondria-14053590) Explain and discuss the structure and functions of the Chloroplasts. Chloroplasts—Chloroplasts, which are found in plants and algae, are the sites of photosynthesis. This process converts solar energy to chemical energy by absorbing sunlight and using it to drive the synthesis of organic compounds such as sugars from carbon dioxide and water. The word chloroplast is derived from the Greek word chloros which means ‘green’ and plastes which means ‘the one who forms’. The chloroplasts are cellular organelles of green plants and some eukaryotic organisms. These organelles conduct photosynthesis. They absorb sunlight and convert it into sugar molecules. They also produce free energy stored in the form of ATP and NADPH through photosynthesis. Chloroplasts are double membrane-bound organelles and are the sites of photosynthesis. The 22 Teacher tip Lecture on mitochondrial membranes can be accessed at (n.d.). Retrieved from <http://www.nature.com/scitable/ topicpage/mitochondria-14053590>. chloroplast has a system of three membranes: the outer membrane, the inner membrane, and the thylakoid system. The outer and the inner membranes of the chloroplast enclose a semi-gel-like fluid known as the stroma. The stroma makes up much of the volume of the chloroplast. The thylakoid system floats in the stroma. Structure of the Chloroplast • • • • • Outer membrane—This is a semi-porous membrane and is permeable to small molecules and ions which diffuse easily. The outer membrane is not permeable to larger proteins. Intermembrane Space—This is usually a thin intermembrane space about 10-20 nanometers and is present between the outer and the inner membrane of the chloroplast. Inner membrane—The inner membrane of the chloroplast forms a border to the stroma. It regulates passage of materials in and out of the chloroplast. In addition to the regulation activity, fatty acids, lipids and carotenoids are synthesized in the inner chloroplast membrane. Stroma—This is an alkaline, aqueous fluid that is protein-rich and is present within the inner membrane of the chloroplast. It is the space outside the thylakoid space. The chloroplast DNA, chloroplast ribosomes, thylakoid system, starch granules, and other proteins are found floating around the stroma. Thylakoid System The thylakoid system is suspended in the stroma. It is a collection of membranous sacks called thylakoids. Thylakoids are small sacks that are interconnected. The membranes of these thylakoids are the sites for the light reactions of the photosynthesis to take place. The chlorophyll is found in the thylakoids. The thylakoids are arranged in stacks known as grana. Each granum contains around 10-20 thylakoids. The word thylakoid is derived from the Greek word thylakos which means 'sack'. Important protein complexes which carry out the light reaction of photosynthesis are embedded in the membranes of the thylakoids. The Photosystem I and the Photosystem II are Teacher tip If an LCD projector is not available, draw the structure of the chloroplast on the board. Lecture on structure and functions of the chloroplast can be accessed at (n.d.). Retrieved from <http:// biology.tutorvista.com/animal-and-plantcells/chloroplasts.html>. complexes that harvest light with chlorophyll and carotenoids. They absorb the light energy and use it to energize the electrons. The molecules present in the thylakoid membrane use the electrons that are energized to pump hydrogen ions into the thylakoid space. This decreases the pH and causes it to become acidic in nature. A large protein complex known as the ATP synthase controls the concentration gradient of the hydrogen ions in the thylakoid space to generate ATP energy. The hydrogen ions flow back into the stroma. PRACTICE (10 MINS) Thylakoids are of two types: granal thylakoids and stromal thylakoids. Granal thylakoids are arranged in the grana. These circular discs that are about 300-600 nanometers in diameter. The stromal thylakoids are in contact with the stroma and are in the form of helicoid sheets. Group the learners into pairs. Ask one to draw the mitochondria and label its parts while the other does the same for chloroplast. Once done, the partners exchange tasks (i.e., the learner that drew the mitochondria now does the same for the chloroplast). The granal thylakoids contain only Photosystem II protein complex. This allows them to stack tightly and form many granal layers with granal membrane. This structure increases stability and surface area for the capture of light. Reproduce these diagrams without the labels and use these for the class activity. To demonstrate how folding increases surface area, ask the learners to trace the edges of the outer membrane with a thread and measure the length of the thread afterwards. Repeat the same for the inner membrane. Compare the results and discuss how the enfolding of the inner membrane increases surface area through folding. The Photosystem I and ATP synthase protein complexes are present in the stroma. These protein complexes act as spacers between the sheets of stromal thylakoids. 24 ENRICHMENT (30 MINS) 1. Using the figure below, ask learners to compute surface area vs. volume. 2. Draw the table on the board and instruct the learners to write their measurements. Teacher tip EVALUATION (60 MINS) Ask the learners to answer practice questions on the following electronic resources: • • • • • Clarify to the learners the misconception that the appearance of organelles are static and rigid. http://www.mcqbiology.com/2013/03/multiple-choice-questions-on_25.html#.Vl7Uq3YrLrc http://www.uic.edu/classes/bios/bios100/summer2004/samples02.htm http://www.tutorvista.com/content/science/science-i/fundamental-unit-life/question-answers-1.php http://www.buzzfeed.com/kellyoakes/the-mitochondria-is-the-powerhouse-of-the-cell#.fajAl0b6o http://global.oup.com/uk/orc/biosciences/cellbiology/wang/student/mcqs/ch10/ Possible responses to the homework (Source: Campbell et al, 10th Ed.): They have double membranes and are not part of the endomembrane system. Their shape is changeable. They are autonomous (somewhat independent) organelles that grow and occasionally pinch in two, thereby reproducing themselves. • They are mobile and move around the cell along tracks of the cytoskeleton, a structural network of the cell. • They contain ribosomes, as well as multiple circular DNA molecules associated with their inner membranes. The DNA in these organelles programs the synthesis of some organelle proteins on ribosomes that have been synthesized and assembled there as well. 2. Give out the homework for next meeting. • • • What are the characteristics shared by these two energy transforming organelles? Instruct the learners to write an essay on probable reasons for these the shared characteristics of the mitochondria and the chloroplast. Learners shall submit a handwritten essay on the Endosymbiotic Theory and how it explains the similarity between the mitochondria and chloroplast. 26 Teacher tip Check the electronic resources on Endosymbiotic Theory: https://www.youtube.com/watch? v=bBjD4A7R2xU (Endosymbiotic Theory in plain English) https://www.youtube.com/watch?v=FQmAnmLZtE EVALUATION Learning Competency Assessment Tool The learners shall be able to describe the following: Learner participation (during lecture) 1. structure and function of major and subcellular organelles (STEM_BIO11/12-Iac-2) Assignment Examination Exemplary Satisfactory Developing Learner was able to answer all the question/ s without referring to his/her notes Learner was able to answer the main question without referring to his/ her notes but was not able to answer follow-up question/s Learner was able to answer the questions but he/ she referred to his/ her notes (1) Learner was not able to answer the question/s Learner submitted an assignment beyond the requirements Learner submitted a comprehensive and wellwritten assignment Learner submitted a well written report but some responses lack details (1) Learner did not submit an assignment Learner obtained 70% to 89.99% correct answers in the examination Learner obtained 50% to 69.99% correct answers in the examination Learner obtained less that 50% correct answers in the examination Learner submitted an essay that was comprehensive and wellwritten Learner submitted a well-written essay some details are lacking (1) Learner did not submit an essay Learner obtained 90% to 100% correct answers in the examination Essay Assignment Learner submitted an essay beyond the requirements Beginnning (2) Learner read notes of his/her classmate (2) Learner submitted a partially-finished assignment (2) Learner submitted a partially-finished essay General Biology 1 180 MINS Structure and Functions of Animal Tissues and Cell Modification LESSON OUTLINE Content Standard The learners demonstrate an understanding of animal tissues and cell modification. Introduction Communicating learning objectives to the learners. Performance Standard Motivation The learners shall be able to construct a three-dimensional model of the animal tissue by using recyclable or indigenous materials. Learning Competencies The learners: Specific Learning Outcomes At the end of the lesson, the learners shall be able to: • 10 Instruction/ Review on the Hierarchy of Biological Organisation and PTSF; Lesson on Animal Delivery 95 Practice Class Activity: Reporting on structure and function of animal tissue or showing of infomercial on diseases. 60 Evaluation Class Quiz 10 Tissues and on Cell Modfication • classify different cell types (plant/animal tissue) and specify the functions of each (STEM_BIO11/12-Ia-c-4) • describe some cell modifications that lead to adaptation to carry out specialized functions (e.g., microvilli, root hair) (STEM_BIO11/12-Ia-c-5) • Class Activity: Pinoy Henyo Classroom Edition 5 Materials present a five-minute report on how the structures of different animal tissues define their function or show a two-minute infomercial about a disease that is caused by animal tissue malfunction; provide insights, offer constructive feedback, and note areas of improvement on their classmates’ reports or infomercial microscopes, LCD Projector (if available), laptop or computer (if available), manila paper, cartolina, photos, images, or illustrations of different types of tissues, drawing materials (e.g. pens, pencils, paper, color pencils, etc.) Resources (continued at the end of Teaching Guide) (1) Reece JB, U. L., (2010). Campbell Biology 10th. San Francisco (CA). 28 INTRODUCTION (5 MINS) Introduce the following learning objectives by flashing these on the board: • • classify different cell types (plant/animal tissue) and specify the functions of each (STEM_BIO11/12Ia-c-4) describe some cell modifications that lead to adaptation to carry out specialized functions (e.g., microvilli, root hair) (STEM_BIO11/12-Ia-c-5) Ask the learners to work in pairs and write the learning objectives using their own words. MOTIVATION (10 MINS) PINOY HENYO CLASSROOM EDITION Divide the class into two groups. Explain to the learners that instead of having the typical one-on-one Pinoy Henyo, only one representative from each group shall be asked to go to the front and have the mystery word card on his/her forehead. Only three words shall be allowed from the groups: “Oo”, “Hindi”, or “Pwede”. Violation of the rules of the game (e.g., communicating the mystery word to the guesser) shall merit corresponding penalties or disqualification. Assign three representatives per group to guess the mystery words. Each guesser shall be given one minute and 30 seconds. At the end of the activity, ask one or two learners what they think the learning objectives of the lesson will be. Immediately proceed with the Introduction. Teacher tip For this particular lesson, start with the Motivation first (i.e., class activity on Pinoy Henyo Classroom Edition). After the game, proceed to the Introduction by communicating the learning objectives to the learners. For the part when the learners have to state the learning objectives using their own words, ask the learners to face their seatmates and work in pairs. If the learners are more comfortable in stating the learning objectives in Tagalog or In their local dialect, ask them to do so. Teacher tip Prior to this lesson, assign a reading material or chapter for this topic. This shall aid in the facilitation of the class activity. In choosing the mystery words for the game, do not limit yourself with the four types of animal tissues. You may choose terms that describe the tissue type or even body parts wherein the tissues are located. You may also include diseases that are caused by certain malfunctions on the tissues. Make sure to mention the chosen mystery words in the discussion. This shall help the learners to understand the connection of the game with the lesson. Check how the class behaves during the activity. If the learners get rowdy, you may choose to stop the game. Make sure to warn the learners of the consequences first before the start of the activity. INSTRUCTION/DELIVERY (95 MINS) Teacher tip Facilitate a five-minute review on the Hierarchy of Biological Organization and on the concept of “form fits function”, the unifying theme in Biology. Review on Hierarchy of Biological Organization Do not use too much time for the review. Just make sure to guide or lead the learners in remembering past lessons. Provide clues if necessary. 1. Discuss that new properties arise with each step upward the hierarchy of life. These are called emergent properties. 2. Ask the class what the levels of biological organization are. The learners should be able to answer this since this is just a review. In case the class does not respond to the question, you may facilitate the discussion by mentioning the first level of the hierarchy. 3. Start with the cell since it is the most basic unit of life that shows all life properties. cells tissue organ organ system multicellular organism Illustrate this by showing photos of the actual hierarchy using animals that are endemic in the Philippines (e.g., pilandok, dugong, and cloud rat). Review on the unifying theme in Biology: “form fits function” 1. Ask the class what the relation of form (structure) to function and vice versa is 2. Ask for examples of versaingit of life that shows all life properthe torpedo shape of the body of dolphins (mammals with fishlike characteristics) and the bone structure and wing shape of birds in relation to flying. 30 Teacher tip For the review on “form fits function”, if the class does not respond well, start giving your own examples for the students to figure out this unifying theme. Make sure to relate structure to function. Mention the role of fossils in determining the habits of extinct animals. By doing this, it shall establish a strong connection between form and function and shall give relevance on the study of this connection in Biology. After this, you may now proceed to the new topic on animal tissues. Facilitate a class activity (i.e., observation of cells under a microscope) to illustrate that animals are made up of cells. This shall be the foundation of the definition of and discussion on animal tissues. The whole activity and discussion shall last for 90 minutes. If microscopes are available for this activity, set up the equipment and the slides that were prepared prior to the activity. Each slide should show one type of tissue (i.e., epithelial tissue, connective tissue, muscle tissue, and nervous tissue). Make sure that the labels are covered because the learners will be asked to name the tissues based on their observations during the discussion. If there are no microscopes available for the activity, prepare cut-out images, photos, or illustrations that show the different types of tissues (i.e., epithelial tissue, connective tissue, muscle tissue, and nervous tissue). Make sure that the images, photos, or illustrations are not labeled because the learners will be asked to name them. Also, do not immediately identify the type of tissue based on the descriptions that you will be presenting to the class. The learners will be asked to identify which among the slides under the microscope or which image, photo, or illustration matches the description of the structure and function that will be given during the discussion. After the class activity, proceed with the actual lecture. If a computer, laptop, or projector is available, show a PowerPoint presentation that shows the description and function of tissues. If there is no available equipment, you may use flash cards or manila paper where description of structure and function of the different tissue types are written down. Ask the learners which among the microscope slides, image, photo, or illustration fits the given information on description and function. After the learners’ responses, you can flash or show the next slide which shall reveal the image of the specimen with the corresponding label or type of tissue. Epithelial Tissue—This type of tissue is commonly seen outside the body as coverings or as linings of organs and cavities. Epithelial tissues are characterized by closely-joined cells with tight junctions (i.e., a type of cell modification). Being tightly packed, tight junctions serve as barriers for pathogens, mechanical injuries, and fluid loss. Teacher tip If microscopes are available for this activity, allot 20-30 minutes for the observation of cells. If microscopes are not available, allot only 10-15 minutes. Prior to the activity, prepare the slides that will be put under the microscopes. The slides shall contain the different types of tissue. Make sure to focus the slides so that the learners can observe them clearly. Give the learners enough time to observe the specimens and then ask them to draw on their notebooks what they were able to observe under the microscopes. Encourage the learners to write down the description and function of the specific tissue type as you go through the discussion. If microscopes are not available and you have shown photos, images, or illustrations instead, ask the learners to draw them on their notebooks and encourage them to write down the description and function of the specific tissue type as you go through the discussion. Teacher tip Prepare the lecture in such a way that you do not immediately reveal the label of the images or the terms that are being described. The learners should first be asked to identify the images or slides that fit the description of the structures and functions. This will make the students more engaged in the discussion. Always remind the learners to take down notes while you flash information for each tissue type. Teacher tip Cells that make up epithelial tissues can have distinct arrangements: • • • • • cuboidal—for secretion simple columnar—brick-shaped cells; for secretion and active absorption simple squamous—plate-like cells; for exchange of material through diffusion stratified squamous—multilayered and regenerates quickly; for protection pseudo-stratified columnar—single layer of cells; may just look stacked because of varying height; for lining of respiratory tract; usually lined with cilia (i.e., a type of cell modification that sweeps the mucus). Figure 1: Epithelial Tissue (Source: Reece JB, U. L. (2010). Campbell Biology 10th. San Francisco (CA):.) 32 Take note that the part on cell modifications is incorporated in the discussion on the structure of the respective cells that make up the tissue that is being discussed. Give emphasis on the differences on the features of the cells that make up the tissue type. For examples or illustrations of the different types of tissues, it is better to use an animal that is endemic in the Philippines or in your specific region so that the learners can relate more in the discussion. Connective Tissue—These tissues are composed of the following: BLOOD —made up of plasma (i.e., liquid extracellular matrix); contains water, salts, and dissolved proteins; erythrocytes that carry oxygen (RBC), leukocytes for defense (WBC), and platelets for blood clotting. CONNECTIVE TISSUE PROPER (CTP)—made up of loose connective tissue that is found in the skin and fibrous connective tissue that is made up of collagenous fibers found in tendons and ligaments. Adipose tissues are also examples of loose connective tissues that store fats which functions to insulate the body and store energy. CARTILAGE —characterized by collagenous fibers embedded in chondroitin sulfate. Chondrocytes are the cells that secrete collagen and chondroitin sulfate. Cartilage functions as cushion between bones. BONE —mineralized connective tissue made by bone-forming cells called osteoblasts which deposit collagen. The matrix of collagen is combined with calcium, magnesium, and phosphate ions to make the bone hard. Blood vessesl and nerves are found at a central canal surrounded by concentric circles of osteons. Figure 2: Connective Tissue (Source: Reece JB, U. L. (2010). Campbell Biology 10th. San Francisco (CA):.) Muscle Tissue—These tissues are composed of long cells called muscle fibers that allow the body to move voluntary or involuntary. Movement of muscles is a response to signals coming from nerve cells. In vertebrates, these muscles can be categorized into the following: • skeletal—striated; voluntary movements • cardiac—striated with intercalated disk for synchronized heart contraction; involuntary • smooth—not striated; involuntary Figure 3: Muscle Tissue (Source: Reece JB, U. L. (2010). Campbell Biology 10th. San Francisco (CA):.) Nervous Tissue—These tissues are composed of nerve cells called neurons and glial cells that function as support cells. These neurons sense stimuli and transmit electrical signals throughout the animal body. Neurons connect to other neurons to send signals. The dendrite is the part of the neuron that receives impulses from other neurons while the axon is the part where the impulse is transmitted to other neurons. Figure 4: Neurons and Glial Cells (Source: Reece JB, U. L. (2010). Campbell Biology 10th. San Francisco (CA):.) PRACTICE (60 MINS) Divide the class into six groups. Four groups will be reporting on Animal Tissues while two groups will be creating an infomercial on diseases caused by the malfunction of tissue types. Each infomercial group shall cover two tissue types. Each group will be given five minutes to report or show their infomercial. At the end of each presentation, facilitate a five-minute critiquing of the presentation. Make sure to get feedbacks from the learners and clarify misconceptions from the reports. The report or the infomercial on diseases shall not be graded. These will be a kind of formative assessment.Group the learners into pairs. Ask one to draw the mitochondria and label its parts while the other does the same for chloroplast. Once done, the partners exchange tasks (i.e., the learner that drew the mitochondria now does the same for the chloroplast). EVALUATION (10 MINS) Ask the learners to group themselves in pairs or in groups of threes. This will allow the learners to discuss and decide among themselves. However, if a learner chooses to do this activity on his or her own, he or she should be allowed to do so. Ask the learners to briefly and clearly answer the following questions: • • • • • • What is the importance of having a tissue level in the hierarchy of biological organization? (2 points) What do the varying shapes and arrangement of epithelial tissue suggests? (2 points) What is the general function of connective tissues? What function is common to all types of connective tissues? (1 point) Why are there voluntary and involuntary muscle tissue functions? (2 points) What is the importance of glial cells in nervous tissues? (1 point) Identify two cell modifications and describe their respective functions. (2 points) Teacher tip Group the learners before starting the lesson. The reporting may be done the day after finishing the discussion on Animal Tissue Structure, Function, and Cell Modification. The reports may be presented using a table which contains columns for tissue type, cell structures that characterize the tissue, part of the body where the tissue is located, function, and importance. Teacher tip Assess if the learners are ready to answer this individually. If they are not yet ready, this activity can be done in pairs or in groups of threes. Make sure that you provide enough time for the group to discuss their responses. Remind the learners to answer briefly and clearly. If you are not comfortable with this time of exam, a multiple-choice type of evaluation may also be prepared. After getting the responses, you may get feedback from the learners to see if all members of each group helped or participated in their small discussions to answer the short quiz. You may ask learners to rate the members of their group. General Biology 1 90 MINS Cell Cycle and Cell Division Content Standard The learners demonstrate an understanding of the cell cycle and cell division (i.e., mitosis and meiosis). LESSON OUTLINE Introduction Presentation of a simplified life cycle of a human being or plant Motivation Performance Standards The learners shall be able to construct a three-dimensional model of the stages or phases involved in the cell cycle using indigenous or recyclable materials. Instruction/ The learners shall put emphasis on the identification of possible errors that may Delivery happen during these stages. Learning Competencies The learners: • • • • • characterize the phases of the cell cycle and their control points (STEM_BIO11/12Id-f-6) describe the stages of mitosis and meiosis given 2n=6 (STEM_BIO11/12-Id-f-7) discuss crossing over and recombination in meiosis (STEM_BIO11/12-Id-f-8) explain the significance or applications of mitosis/meiosis (STEM_BIO11/12-Id-f-9) identify disorders and diseases that result from the malfunction of the cell during the cell cycle (STEM_BIO11/12-Id-f-10) Specific Learning Outcomes • Identify and differentiate the phases of the cell cycle and their control points • describe and differentiate the stages of mitosis and meiosis given 2n=6 • discuss and demonstrate crossing over and recombination in meiosis • explain the significance and applications of mitosis and meiosis • construct a diagram of the various stages of mitosis and meiosis • identify disorders and diseases that result from malfunctions in the cell during the cell cycle Practice Video presentation of ‘Cell Cycle and Cell Division’ 60 Class activities or games such as Amazing Race or Interphase, Mitosis, or Meiosis Puzzle 10 and animal gametogenesis; Microscopic examination of an onion root tip Written or oral examination 5 5 Materials photos of the life cycle or stages of eukaryotic organisms, yarns of different thickness, cords, beads, coins, pens Resources (continued at the end of Teaching Guide) (1) Becker, W.M. (2000). The World of the Cell. Addison Wesley Longman Inc., USA (2) Mader, S.S. (2011).Biology 10th Ed. Mac Graw Hill Education, USA. 36 5 Lecture-discussion on the cell through the use of a PowerPoint presentation, video, or cell diagram on a Manila paper; Demonstration of the processes inside the cell using model materials (e.g., beads, cords, yarn with different thickness, coins, etc.); or, Summary of learners’ responses to questions regarding the video on ‘Cell Cycle and Cell Division’ Enrichment Video presentation or introduction on plant Evaluation 5 INTRODUCTION (5 MINS) Teacher tip Introduce a simplified life cycle of a human being or plant. Let the learners identify the changes throughout the different stages and how these organisms grow and develop. Explain to the learners that these eukaryotic organisms follow a complex sequence of events by which their cells grow and divide. This sequence of events is known as the Cell Cycle. You can show diagrams or illustrations that demonstrate the growth or increase in the number of organisms. Figure 1: Life Cycle of Man and Higher Plants (Source: (n.d.). Retrieved from http:// www.vcbio.science.ru.nl/en/virtuallessons/cellcycle/postmeio/) MOTIVATION (5 MINS) 1. Play the video on ‘Cell Cycle and Cell Division’. This video can be accessed at http:// www.youtube.com/watch?v=Q6ucKWIIFmg.Divide the class into two groups. 2. Show diagrams of cell division in multicellular or eukaryotic organisms to the class. 38 Teacher tip You can download the video prior to this session or if internet connection is available during class, you can just make use of the hyperlink to play the video. To access the video through the hyperlink, simply hold the Control (Ctrl) Key on the keyboard and click on the hyperlink. You should ask the learners thoughtprovoking questions about the video and relate it to the lesson. INSTRUCTION/DELIVERY (30 MINS) Teacher tip Facilitate a lecture-discussion on the general concepts of cell division. Cell Division—involves the distribution of identical genetic material or DNA to two daughter cells. What is most remarkable is the fidelity with which the DNA is passed along, without dilution or error, from one generation to the next. Cell Division functions in reproduction, growth, and repair. Core Concepts: • • • • • • • • • • All organisms consist of cells and arise from preexisting cells. Mitosis is the process by which new cells are generated. Meiosis is the process by which gametes are generated for reproduction. The Cell Cycle represents all phases in the life of a cell. DNA replication (S phase) must precede mitosis so that all daughter cells receive the same complement of chromosomes as the parent cell. The gap phases separate mitosis from S phase. This is the time when molecular signals mediate the switch in cellular activity. Mitosis involves the separation of copied chromosomes into separate cells. Unregulated cell division can lead to cancer. Cell cycle checkpoints normally ensure that DNA replication and mitosis occur only when conditions are favorable and the process is working correctly. Mutations in genes that encode cell cycle proteins can lead to unregulated growth, resulting in tumor formation and ultimately invasion of cancerous cells to other organs. The Cell Cycle control system is driven by a built-in clock that can be adjusted by external stimuli (i.e., chemical messages). Checkpoint—a critical control point in the Cell Cycle where ‘stop’ and ‘go-ahead’ signals can regulate the cell cycle. • • Animal cells have built-in ‘stop’ signals that halt the cell cycles and checkpoints until overridden by ‘go-ahead’ signals. Three major checkpoints are found in the G1, G2, and M phases of the Cell Cycle. 38 Note the learners’ responses to questions about the video compared to the expected responses. The expected responses are the concepts listed in the Instruction / Delivery part. The G1 Checkpoint—the Restriction Point • • • • The G1 checkpoint ensures that the cell is large enough to divide and that enough nutrients are available to support the resulting daughter cells. If a cell receives a ‘go-ahead’ signal at the G1 checkpoint, it will usually continue with the Cell Cycle. If the cell does not receive the ‘go-ahead’ signal, it will exit the Cell Cycle and switch to a non-dividing state called G0. Most cells in the human body are in the G0 phase. The G2 Checkpoint—ensures that DNA replication in S phase has been successfully completed. The Metaphase Checkpoint—ensures that all of the chromosomes are attached to the mitotic spindle by a kinetochore. Kinase—a protein which activates or deactivates another protein by phosphorylating them. Kinases give the ‘go-ahead’ signals at the G1 and G2 checkpoints. The kinases that drive these checkpoints must themselves be activated. • • • • • • The activating molecule is a cyclin, a protein that derives its name from its cyclically fluctuating concentration in the cell. Because of this requirement, these kinases are called cyclin-dependent kinases or CDKs. Cyclins accumulate during the G1, S, and G2 phases of the Cell Cycle. By the G2 checkpoint, enough cyclin is available to form MPF complexes (aggregations of CDK and cyclin) which initiate mitosis. MPF functions by phosphorylating key proteins in the mitotic sequence. Later in mitosis, MPF switches itself off by initiating a process which leads to the destruction of cyclin. CDK, the non-cyclin part of MPF, persists in the cell as an inactive form until it associates with new cyclin molecules synthesized during the interphase of the next round of the Cell Cycle. Discuss the stages of mitosis and meiosis. Mitosis (apparent division)—is nuclear division; the process by which the nucleus divides to produce two new nuclei. Mitosis results in two daughter cells that are genetically identical to each other and to the parental cell from which they came. Cytokinesis—is the division of the cytoplasm. Both mitosis and cytokinesis last for around one to two hours. Prophase—is the preparatory stage, During prophase, centrioles move toward opposite sides of the nucleus. • The initially indistinct chromosomes begin to condense into visible threads. • Chromosomes first become visible during early prophase as long, thin, and intertwined filaments but by late prophase, chromosomes are more compacted and can be clearly discerned as much shorter and rod-like structures. • As the chromosomes become more distinct, the nucleoli also become more distinct. By the end of prophase, the nucleoli become less distinct, often disappearing altogether. Metaphase—is when chromosomes become arranged so that their centromeres become aligned in one place, halfway between the two spindle poles. The long axes of the chromosomes are 90 degrees to the spindle axis. The plane of alignment is called the metaphase plate. Anaphase—is initiated by the separation of sister chromatids at their junction point at the centromere. The daughter chromosomes then move toward the poles. Telophase—is when daughter chromosomes complete their migration to the poles. The two sets of progeny chromosomes are assembled into two-groups at opposite ends of the cell. The chromosomes uncoil and assume their extended form during interphase. A nuclear membrane then forms around each chromosome group and the spindle microtubules disappear. Soon, the nucleolus reforms. Meiosis—reduces the amount of genetic information. While mitosis in diploid cells produces daughter cells with a full diploid complement, meiosis produces haploid gametes or spores with only one set of chromosomes. During sexual reproduction, gametes combine in fertilization to reconstitute the diploid complement found in parental cells. The process involves two successive divisions of a diploid nucleus. First Meiotic Division The first meiotic division results in reducing the number of chromosomes (reduction division). In most cases, the division is accompanied by cytokinesis. 40 Teacher tip You may show diagrams or a video demonstrating animal and plant mitosis. The video can be accessed at http:// www.vcbio.science.ru.nl/en/virtuallessons/ mitostage/ Prophase I—has been subdivided into five substages: leptonema, zygonema, pachynema, diplonema, and diakinesis. • • • • • Leptonema—Replicated chromosomes have coiled and are already visible. The number of chromosomes present is the same as the number in the diploid cell. Zygonema—Homologue chromosomes begin to pair and twist around each other in a highly specific manner. The pairing is called synapsis. And because the pair consists of four chromatids it is referred to as bivalent tetrad. Pachynema—Chromosomes become much shorter and thicker. A form of physical exchange between homologues takes place at specific regions. The process of physical exchange of a chromosome region is called crossing-over. Through the mechanism of crossing-over, the parts of the homologous chromosomes are recombined (genetic recombination). Diplonema—The two pairs of sister chromatids begin to separate from each other. It is at this point where crossing-over is shown to have taken place. The area of contact between two non-sister chromatids, called chiasma, become evident. Diakinesis—The four chromatids of each tetrad are even more condensed and the chiasma often terminalize or move down the chromatids to the ends. This delays the separation of homologous chromosomes. In addition, the nucleoli disappear, and the nuclear membrane begins to break down. Metaphase I—The spindle apparatus is completely formed and the microtubules are attached to the centromere regions of the homologues. The synapsed tetrads are found aligned at the metaphase plate (the equatorial plane of the cell) instead of only replicated chromosomes. Anaphase I—Chromosomes in each tetrad separate and migrate toward the opposite poles. The sister chromatids (dyads) remain attached at their respective centromere regions. Telophase I—The dyads complete their migration to the poles. New nuclear membranes may form. In most species, cytokinesis follows, producing two daughter cells. Each has a nucleus containing only one set of chromosomes (haploid level) in a replicated form. Second Meiotic Division The events in the second meiotic division are quite similar to mitotic division. The difference lies, however, in the number of chromosomes that each daughter cell receives. While the original chromosome number is maintained in mitosis, the number is reduced to half in meiosis. Prophase II—The dyads contract. Metaphase II—The centromeres are directed to the equatorial plate and then divide. Anaphase II—The sister chromatids (monads) move away from each other and migrate to the opposite poles of the spindle fiber. Telophase II—The monads are at the poles, forming two groups of chromosomes. A nuclear membrane forms around each set of chromosomes and cytokinesis follows. The chromosomes uncoil and extend. Cytokinesis—The telophase stage of mitosis is accompanied by cytokinesis. The two nuclei are compartmentalized into separate daughter cells and complete the mitotic cell division process. In animal cells, cytokinesis occurs by the formation of a constriction in the middle of the cell until two daughter cells are formed. The constriction is often called cleavage, or cell furrow. However, in most plant cells this constriction is not evident. Instead, a new cell membrane and cell wall are assembled between the two nuclei to form a cell plate. Each side of the cell plate is coated with a cell wall that eventually forms the two progeny cells. Meiosis Teacher tip You can show a tabular comparison between mitosis and meiosis to point the significance of the two types of division. Divide the class into two groups and ask them about their opinions on the applications of mitosis and meiosis. The following could be possible responses: Mitosis 1. Requires two nuclear divisions 1. Requires one nuclear division 2. Chromosomes synapse and cross over 2. Chromosomes do not synapse nor cross over 3. Centromeres survive Anaphase I 3. Centromeres dissolve in mitotic anaphase 4. Halves chromosome number 4. Preserves chromosome number 5. Produces four daughter nuclei 5. Produces two daughter nuclei 6. Produces daughter cells genetically different from parent and each other 6. Produces daughter cells genetically identical to parent and to each other 7. Used only for sexual reproduction 7. Used for asexual reproduction and growth Table 1: Comparison of Mitosis and Meiosis (Source: http://courses.washington.edu/bot113/spring/ WebReadings/PdfReadings/TABLE_COMPARING_MITOSIS_AND.pdf) 42 Significance of mitosis for sexual reproduction: Mitosis is important for sexual reproduction indirectly. It allows the sexually reproducing organism to grow and develop from a single cell into a sexually mature individual. This allows organisms to continue to reproduce through the generations. Significance of Meiosis and Chromosome Number: Chromosomes are the cell's way of neatly arranging long strands of DNA. Non-sex cells have two sets of chromosomes, one set from each parent. Meiosis makes sex cells with only one set of chromosomes. For example, human cells have 46 chromosomes, with the exception of sperm and eggs, which contain only 23 chromosomes each. When a sperm cell fertilizes an egg, the 23 chromosomes from each sex cell combine to make a zygote, a new cell with 46 chromosomes. The zygote is the first cell in a new individual. Meiosis I compared to Mitosis Meiosis II compared to Mitosis Meiosis I Mitosis Meiosis II Mitosis Prophase I Prophase Prophase II Prophase Pairing of homologous chromosomes No pairing of chromosomes No pairing of chromosomes No pairing of chromosomes Metaphase I Metaphase Metaphase II Metaphase Bivalents at metaphase plate Duplicated chromosomes at metaphase plate Haploid number of duplicated chromosomes at metaphase plate Diploid number of duplicated chromosomes at metaphase plate Anaphase I Anaphase Anaphase II Anaphase Homologues of each bivalent separate and duplicated chromosomes move to poles Sister chromatids separate, becoming daughter chromosomes that move to the poles Sister chromatids separate, becoming daughter chromosomes that move to the poles Sister chromatids separate becoming daughter chromosomes that move to the poles Telophase I Telophase Telophase II Telophase Two haploid daughter cells not identical to the parent cell Two diploid daughter cells, identical to the parent cell Four haploid daughter cells not genetically identical Two diploid daughter cells, identical to the parent cell Table 2: Meiosis compared to Mitosis Facilitate a discussion on disorders and diseases that result from the malfunction of the cell during the cell cycle. Present some diagrams or illustrations on some errors in mitosis and allow the learners to predict possible outcomes, diseases, or disorders that may happen: • • incorrect DNA copy (e.g., cancer) chromosomes are attached to string-like spindles and begin to move to the middle of the cell (e.g., Down Syndrome, Alzheimer’s, and Leukemia) Teacher tip Significance of Meiosis for Diversity: One of the benefits of sexual reproduction is the diversity it produces within a population. That variety is a direct product of meiosis. Every sex cell made from meiosis has a unique combination of chromosomes. This means that no two sperm or egg cells are genetically identical. Every fertilization event produces new combinations of traits. This is why siblings share DNA with parents and each other, but are not identical to one another. Teacher tip You may show a video that demonstrates how crossing over and recombination of chromosomes occur. The video can be accessed at http:// highered.mheducation.com/sites/ 9834092339/student_view0/chapter11/ meiosis_with_crossing_over.html.s Other chromosome abnormalities: • • • • arise from errors in meiosis, usually meiosis I; occur more often during egg formation (90% of the time) than during sperm formation; become more frequent as a woman ages. Aneuploidy—is the gain or loss of whole chromosomes. It is the most common chromosome abnormality. It is caused by non-disjunction, the failure of chromosomes to correctly separate: • homologues during meiosis I or • sister chromatids during meiosis II PRACTICE (10 MINS) Facilitate games like Amazing Race, Interphase/Mitosis/Meiosis Puzzle in the class. 1. The Amazing Race follows a series of stations or stages with challenges that the learners have to accomplish. Divide the class into groups after the discussion. The number of groups will depend on the number of stages or phases in the process (i.e., interphase, mitosis, or meiosis). Teacher tip 2. The groups will race to accomplish the tasks in five stations. In each station, the learners will assemble given materials to illustrate stages or phases of events in the specific process (i.e., interphase, mitosis, or meiosis). Encourage the learners to actively participate in the challenge. You may give extra points to those who will finish first. ENRICHMENT (5 MINS) A number of good videos have the stages or phases made into a rap or a song. One such example is the video entitled Cell Division Song Spongebob that can be accessed at http://www.youtube.com/watch? v=9nsRufogdoI. Encourage each group to brainstorm and point out their perceptions of the videos. 1. Instruct the learners to watch additional videos on cell division. 2. Introduce animal and plant gametogenesis to the learners in order for them to appreciate the significance of cell division. 3. Facilitate microscopic examination of onion root tip. EVALUATION (5 MINS) Facilitate the accomplishment of a self-assessment checklist. A video on animal and plant gametogenesis can be accessed at http://csls-text.c.u-tokyo.ac.jp/active/ 12_05.html. 44 ADDITIONAL RESOURCES: Books: 1. Raven, P. a. (2001). Biology 6th Ed. The McGraw Hill Company, USA 2. Reece, J. B. (2013). Campbell Biology, 10th Ed. Pearson Education, Inc. United States of America. Electronic Resources: 3. (n.d.). Retrieved from Bright Hub Education: http://www.brighthubeducation.com/middle-school-science-lessons/94267-three-activities-forteaching-cell-cycles/# 4. (n.d.). Retrieved from http://www.uic.edu/classes/bios/bios100/lecturesf04am/lect16.htm 5. (n.d.). Retrieved from MH Education: http://highered.mheducation.com/sites/9834092339/student_view0/chapter11/ meiosis_with_crossing_over.html 6. (n.d.). Retrieved from http://www.vcbio.science.ru.nl/en/virtuallessons/meiostage/ 7. (n.d.). Retrieved from http://csls-text.c.u-tokyo.ac.jp/active/12_05.html 8. (n.d.). Retrieved from http://education.seattlepi.com/biological-significance-mitosis-meiosis-sexual-reproduction-5259.htm General Biology 1 480 MINS Transport Mechanisms Pt.1 LESSON OUTLINE Content Standards The learners demonstrate an understanding of Transport Mechanisms: Introduction Visualization of the plasma membrane and its Simple Diffusion, Facilitated Transport, Active Transport, and Bulk/Vesicular Transport Motivation Simple group activity and brief reporting Performance Standards The learners shall be able to construct a cell membrane model from indigenous Instruction/ Discussion and lecture proper Delivery or recyclable materials. Learning Competencies The learners: • • • • Practice describe the structural components of the cell membrane (STEM_BIO11/12–Ig-h-11) relate the structure and composition of the cell membrane to its function (STEM_BIO11/12-Ig-h-12) explain transport mechanisms in cells (diffusion, osmosis, facilitated transport, active transport) (STEM_BIO11/12–Ig-h-13) differentiate exocytosis and endocytosis (STEM_BIO11/12-Ig-h-14) Specific Learning Outcomes At the end of the lesson, the learners shall be able to: • • • • 30 functions 60 120 45 Answering practice/guide questions Enrichment Essay and concept map writing Evaluation Designing a model of a plasma membrane using recyclable or indigenous materials 45 180 Materials pen, paper, salt, water, recycled or indigenous materials describe and compare diffusion, osmosis, facilitated transport and active transport explain factors that affect the rate of diffusion across a cell membrane predict the effects of hypertonic, isotonic, and hypotonic environments on osmosis in animal cells differentiate endocytosis (phagocytosis and pinocytosis) and exocytosis 46 Resources (1) Campbell, N. J. (n.d.). (2) Campbell, N. e. (2008). Biology 8th edition. Pearson International Edition. Pearson/Benjamin. (3) Freeman, S. (2011). Biological Science 4th edition International Edition. Benjamin Cummings Publishing. (4) Hickman, C. L. (2011). Integrated Principles of Zoology 15th edition. McGraw Hill Co., Inc. INTRODUCTION (30 MINS) 1. Before this lesson, ask the learners to read about the topic on transport of materials across membranes. 2. Introduce the topic by providing the learners with background information. In order for the cell to stay alive, it must meet the characteristics of life which include taking nutrients in and eliminating wastes and other by-products of metabolism. Several mechanisms allow cells to carry out these processes. All of the cell’s activities are in one way or another tied to the membrane that separates its interior from the environment. 3. Ask the learners how they understand and visualize a plasma membrane and what characteristics are essential for it to perform its function. 4. Ask the learners to identify the different mechanisms on how materials are transported in and out of the cell. MOTIVATION (60 MINS) 1. Divide the learners into groups and ask them the following question: “What comes to your mind when you see a 20 year old man who is 7.5 ft. tall and 3.5 ft. tall man of the same age?” Among their respective groups, let the learners discuss the similarities and differences between the two. (Hint: Give students a clue by giving them the giant and pygmy as examples). 2. Ask a representative from each group to report the result of their discussion to the whole class. 3. Before the start of the lesson on diffusion, spray an air freshener in one corner of the room and ask the learners to raise their hands if they have smelled the scent of the spray. 4. Ask the learners what they have observed. Who smelled the scent first? Who are the last ones to smell the scent? How would you explain the phenomenon wherein learners in the same classroom smelled the spray at different times? INSTRUCTION/DELIVERY (120 MINS) 1. Show an illustration of a plasma membrane to the learners. 2. Ask the learners to describe the plasma membrane. 3. Discuss the importance of the plasma membrane and how indispensable it is to the life of the cell. 4. Explain how plasma membranes are arranged in the presence of water. 5. Let the learners enumerate the structures found in a plasma membrane. Teacher tip Different responses to the question will be drawn from students. Their responses will depend on what aspect they are looking into. Acknowledge the responses of the learners. Point out and explain that the two men are both abnormal. Their growths are abnormal such that one is too big in size and the other one is too small. Both men have defective membranes. Insufficient amount of growth hormones pass through a pygmy’s body while an excessive amount of growth hormones is released in a giant. 6. Explain to the learners the structure of a phospholipid bilayer. Phospholipids are the foundation of all known biological membranes. The lipid bilayer forms as a result of the interaction between the nonpolar phospholipid tails, the polar phospholipid heads, and the surrounding water. The nonpolar tails face toward the water. Transmembrane proteins float within the bilayer and serve as channels through which various molecules can pass. 7. Ask the learners to enumerate the different transport mechanisms. interior to accommodate the natural inward movement. Most plants are hypertonic with respect to their immediate environment. Osmotic pressure within the cell pushes the cytoplasm against the cell wall and makes a plant cell rigid. 8. Differentiate between diffusion and osmosis. 9. Compare and contrast facilitated diffusion and active transport. 10. Present photos of plant and animal cells immersed in an isotonic, hypotonic, and hypertonic solution. To control the entrance and exit of particular molecules, selective transport of materials is necessary. One simple process is facilitated diffusion that utilizes protein transmembrane channels that are specific to certain molecules. It is a passive process driven by the concentration of molecules both inside and the outside of the membrane. Certain molecules are transported in and out of the cell, independent of concentration. This process requires the expenditure of energy in the form of ATP and is called active transport. 11. Describe solution and solute movement in and out of the cell under hypertonic, hypotonic, and isotonic conditions. 12. Explain the effects of the different solutions to the cells. Ask which among the three solutions is the best for plants? How about for animals? Explain to the learners the water requirement in plants. Diffusion is the natural tendency for molecules to move constantly. Their movement is random and is due to the energy found in the individual molecules. Net diffusion occurs when the materials on one side of the membrane have a different concentration than the materials on the other side. 13. Differentiate among endocytosis, phagocytosis, pinocytosis, receptor-mediated endocytosis, and exocytosis. Large molecules enter the cell by generalized nonselective process known as endocytosis. Phagocytosis is endocytosis of a particulate material while endocytosis of liquid material is called pinocytosis. Exocytosis is the reverse process. Receptormediated endocytosis is a complicated mechanism involving the transport of materials via coated vesicles. Osmosis is a special type of diffusion specifically associated with the movement of water molecules. Many cells are isotonic to the environment to avoid excessive inward and outward movement of water. Other cells must constantly export water from their 48 PRACTICE (45 MINS) Ask the learners to answer the following practice or guide questions: • • • • What is the difference between diffusion and facilitated diffusion? How do endocytosis and exocytosis allow movement of materials in and out of the cell? What solution is best for a plant cell? How about for an animal cell? Explain the orientation of the phospholipid molecules in the presence of water. ENRICHMENT (45 MINS) Let the learners recognize the effect of a defective membrane in normal body functioning. Ask them to write an essay about the possible effects of a faulty plasma membrane aside from the examples given earlier. Ask the learners to individually submit a concept map about plasma membrane and the different transport mechanisms. EVALUATION (180 MINS) Ask the learners to design and a model of a plasma membrane using recyclable or indigenous materials. Divide the learners into groups and assign different concentrations of salt solution to be used in making salted eggs. Ask the learners to answer the following questions: • • Why does putting salt on meat preserve it from bacterial spoilage? Compare specific transport processes (i.e., diffusion, osmosis, facilitated transport, active transport, endocytosis, and exocytosis) in terms of the following: • concentration gradient • use of channel or carrier protein • use of energy • types or sizes of molecules transported General Biology 1 240 MINS Transport Mechanisms Pt.2 LESSON OUTLINE Content Standard Introduction Presentation of objectives and important terms; Discussion on the structure of the plasma The learners shall be able to construct a cell membrane model from indigenous membrane; Brief discussion on the different or recyclable materials. 15 transport mechanisms Performance Standard The learners shall be able to construct a cell membrane model from indigenous Motivation or recyclable materials. Learning Competencies The learners: • • • • describe the structural components of the cell membrane (STEM_BIO11/12–Ig-h-11) relate the structure and composition of the cell membrane to its function (STEM_BIO11/12-Ig-h-12) explain transport mechanisms in cells (diffusion, osmosis, facilitated transport, active transport) (STEM_BIO11/12–Ig-h-13) differentiate exocytosis and endocytosis (STEM_BIO11/12-Ig-h-14) Specific Learning Outcomes At the end of the lesson, the learners shall be able to: • • • • • • • describe the plasma membrane explain how plasma membranes are arranged in the presence of water understand the structure of the phospholipid bilayer describe and compare diffusion, osmosis, facilitated transport and active transport explain factors that affect the rate of diffusion across a cell membrane predict the effects of hypertonic, isotonic, and hypotonic environments on osmosis in animal cells differentiate endocytosis (phagocytosis and pinocytosis) and exocytosis Class activity to illustrate the process of diffusion; Discussion of similarities between a giant and pygmy; Demonstration of the principle behind the process of making salted eggs 15 Instruction/ Delivery Discussions, as a class and among groups, on the structure and importance of the plasma membrane and on the different transport mechanisms 60 Practice Answering of practice or guide questions 30 Enrichment Essay writing or concept mapping; Class activity on salted egg making 60 Evaluation Construction of a plasma membrane model from indigenous or recyclable materials; Concept mapping on the different transport mechanisms; Answering of questions for assessment 60 Materials projector, laptop (if available), visual aids, school supplies, recycled or indigenous materials Resources (1) Campbell, N.A. et. al. (2008). Biology 8th Edition Pearson International. Pearson/Benjamin Cummings Publishing. (2) Campbell, N. J. (2010). Biology 9th edition Pearson International Edition. Benjamin Cummings Publishing. (3) Freeman, S. (2011). Biological Science. 4th edition. International Edition. Benjamin Cummings Publishing. (4) Hickman, C. L. (2011). Integrated Principles of Zoology. 15th edition. McGraw Hill Co., Inc. 50 INTRODUCTION (15 MINS) Prior to this lesson, instruct the learners to read up on the transport of materials across membranes. Ask the learners to identify the different mechanisms on how materials are transported in and out of the cell. Introduce the topic by providing the learners with background information. In order for the cell to stay alive, it must meet the characteristics of life which include taking nutrients in and eliminating wastes and other by-products of metabolism. Several mechanisms allow cells to carry out these processes. All of the cell’s activities are, in one way or another, tied to the membrane that separates its interior from the environment. Ask the learners how they visualize a plasma membrane and what characteristics do they think are essential for it to perform its function. MOTIVATION (15 MINS) Before the start of the lesson on diffusion, conduct this simple class activity. Spray an air freshener in one corner of the room and instruct the learners to raise their hands if they have smelled the scent of the spray. Ask the learners the following questions: • Who among the class were able to smell the air freshener first? • Who among the class were the last ones to smell the air freshener? • How would you explain the phenomenon wherein people in the same classroom smelled the scent of the air freshener at different times? Divide the learners into groups and ask them the question: What comes to your mind when you see two men who are of the same age but one is 7.5 feet tall and the other is 3.5 feet tall? Allow the learners to discuss the similarities and differences between the two among their groups. Ask a representative from each group to present the results of their discussions to the whole class. Teacher tip After the learners have enumerated the different transport mechanisms, ask them why they think there is a need to have different kinds of processes that allow materials to be transported in and out of the cell. Learners will describe the plasma membrane in different ways. Ask them how they think the structures found within the membrane help in performing its function and what might happen in the absence of the these structures Teacher tip Allow some time for the learners to smell the spray until everyone has already smelled the scent. Remember to instruct the learners to raise their hand once they smell the scent. The learners might give varying responses to the question depending on what aspect they are looking into. Give hints by providing the giant and pygmy as examples. Acknowledge the learners’ responses and point out that the two men are similar in the sense that they are both abnormal. Growth in both men is abnormal such that one is too big in size while the other one is too small. Explain that both men have abnormal growth. Both have defective membranes. Insufficient amount of growth hormones pass through a pygmy’s body while an excessive amount of growth hormones is released in a giant. INSTRUCTION/DELIVERY (60 MINS) Teacher tip Structure, function and importance of the plasma membrane 1. Present an illustration of the plasma membrane to the class 2. Ask the learners to describe the plasma membrane. 3. Discuss the importance of the plasma membrane and how indispensable it is to the life of the cell. 4. Explain how plasma membranes are arranged in the presence of water. 5. Let students enumerate structures found in a plasma membrane. 6. Make students understand the structure of a phospholipid bilayer. Plasma membranes—are made up of a phospholipid bilayer in an aqueous environment. Phospholipids are the foundation of all known biological membranes. The lipid bilayer forms as a result of the interaction between the non-polar (hydrophobic or water-fearing) phospholipid tails, the polar (hydrophilic or water-loving) phospholipid heads, and the surrounding water. The nonpolar tails face toward the water. Transmembrane proteins float within the bilayer and serve as channels through which various molecules can pass. They function as ‘identification tags’ on cells which enable the cell to determine if the other cells that it encounters are like itself or not. It also permits cells of the immune system to accept and reject foreign cells such as disease-causing bacteria. Many membrane proteins function as enzymes that speed up reactions in cells. Others act like paste or glue-forming cell junctions where adjacent cells stick together. Membranes also contain cholesterol which reduces the cell’s permeability to substances and make the bilayer stronger. Transport Mechanisms 1. Ask the learners to enumerate the different transport mechanisms. 2. Differentiate between diffusion and osmosis. 52 You can ask the following questions before starting the discussion: Have you realized how crucial the task of a plasma membrane is in maintaining the life of a cell? Have you thought about the ways on how the materials needed by the cell and the wastes it needs to dispose are able to move in and out of the plasma membrane? Molecules and substances move in several ways that fall within two categories: passive transport and active transport. In passive transport, heat energy of the cellular environment provides all of the energy, hence, this is not energy-costly to the cell. Active transport, however, requires the cell to do work, requiring the cell to expend its energy reserves. Diffusion is a type of passive transport described as the natural tendency for molecules to move constantly. Their movement is random and is due to the energy found in the individual molecules. Net diffusion occurs when the materials on one side of the membrane have a different concentration than the materials on the other side. Osmosis is a special type of diffusion specifically associated with the movement of water molecules. A solution with a higher concentration of solutes is said to be hypertonic while a solution with a lower concentration of solutes is hypotonic. Water crosses the membrane until the solute concentrations are equal on both sides. Solutions of equal solution concentration are said to be isotonic. This only occurs when the solute concentration are the same on both sides of the membrane. Compare and contrast facilitated diffusion and active transport. Then present photos of plant and animal cells immersed in an isotonic, hypotonic, and hypertonic solution. In addition, describe a solution and solute movement into and out of the cell under hypertonic, hypotonic and isotonic conditions. Explain the effects of the different solutions to the cells. Ask which among the three solutions is the best for plants? For animals? Let them understand water requirement in plants. Many cells are isotonic to the environment in order to avoid excessive inward and outward movement of water. Other cells must constantly export water from their interior to accommodate the natural inward movement. Most plants are hypertonic with respect to their immediate environment. Osmotic pressure within the cell pushes the cytoplasm against the cell wall and makes a plant cell rigid. Ask the learners the following questions: • • • How do cells behave in different solutions? What do you notice about the effect of different solutions to animal and plant cells? What solution is best for an animal cell? Does this hold true with plant cells? When an animal cell such as red blood cell is immersed in an isotonic solution, the cell gains water at the same rate that it loses it. The cell’s volume remains constant in this situation. What will happen to the red blood cell when immersed in a hypotonic solution which has a lower solute concentration than the cell? The cell gains water, swells, and may eventually burst due to excessive water intake. When placed in a hypertonic solution, an animal cell shrinks and can die due to water loss. Water requirement for plant cells is different due to their rigid cell walls. A plant cell placed in an isotonic solution is flaccid and a plant wilts in this condition. In contrast with animal cells, a plant cell is turgid and healthy in a hypotonic solution. In a hypertonic solution, a plant cell loses water, shrivels, and its plasma membrane detaches from the cell wall. This situation eventually causes death in plant cells. Differentiate diffusion from facilitated diffusion. To control the entrance and exit of particular molecules, selective transport of materials is necessary. One simple process is facilitated diffusion that utilizes protein transmembrane channels that are specific to certain molecules. It is a passive process driven by the concentration of molecules on the inside and the outside of the membrane. Certain molecules are transported in and out of the cell, independent of concentration. This process requires the expenditure of energy in the form of ATP and is called active transport. Differentiate endocytosis, phagocytosis, pinocytosis, receptor-mediated endocytosis, and exocytosis. Large molecules enter the cell by generalized non-selective process known as endocytosis. Phagocytosis is endocytosis of a particulate material while pinocytosis is endocytosis of liquid material. In this process, the plasma membrane engulfs the particle or fluid droplet and pinches off a membranous sac or vesicle with a particular fluid inside into the cytoplasm. Exocytosis is the reverse process where a membrane-bound vesicle filled with bulky materials moves to the plasma membrane and fuses with it. In this process, the vehicle’s contents are released out of the cell. Receptor-mediated endocytosis is a complicated mechanism involving the transport of materials through coated vesicles. Cells take up molecules more efficiently in this process due to the receptor proteins on their surfaces. Each receptor protein bears a binding site for a particular molecule. If the right molecule contacts a receptor protein, it attaches to the binding site, forming a pocket and eventually pinching off into the cytoplasm. PRACTICE (30 MINS) Ask the learners to answer the following questions: • • Explain the orientation of the phospholipid molecules in the presence of water. Enumerate the structures found in a plasma membrane and give the function of each. 54 • • • • • • How do diffusion and facilitated diffusion differ? How do endocytosis and exocytosis allow movement of materials in and out of the cell? What solution is best for a plant cell? How about for an animal cell? Give two ways by which one could determine whether active transport is going on. Compare and contrast the effects of hypertonic and hypotonic solutions on plant and animal cells. What role do vacuoles play in endocytosis and exocytosis? ENRICHMENT (60 MINS) Essay writing and concept mapping 1. Ask the learners to write an essay about the possible effects of a faulty plasma membrane aside from the examples given in the lesson. Let the learners recognize the effects of a defective membrane to normal bodily functions. 2. Ask the learners to individually submit a concept map about the plasma membrane. You can provide them with sample words for their concept map: • • • • • • • plasma membrane semipermeable phospholipid bilayer hydrophilic heads hydrophobic tails cholesterol membrane proteins Teacher tip For the concept mapping, you can provide the learners with key words or allow them to come up with their own key words for their concept map. Creating own saturated salt solution for salted egg-making 1. Divide the class into groups and assign different concentrations of salt solutions to be used in making salted eggs. 2. Instruct the learners to make their own salt solutions and take note of the concentration that they opt to use. Teacher tip Diffusion and osmosis are two processes involved in making salted eggs. The salt solution should be supersaturated in order to produce good and delicious salted eggs. EVALUATION (60 MINS) Building of plasma membrane model 1. Divide the class into groups. 2. Ask the groups to design and build a model of a plasma membrane using recyclable or indigenous materials. Concept mapping Ask the learners to individually submit a concept map about the different transport mechanisms. You can provide them with sample words for their concept map or allow them to come up with their own: • plasma membrane • phagocytosis • transport mechanisms • pinocytosis • passive transport • receptor-mediated endocytosis • active transport • diffusion • hypotonic • facilitated diffusion • hypertonic • endocytosis • isotonic • exocytosis Assessment questions: Instruct the learners to answer the following questions to assess their knowledge and understanding of the lesson: • • Why does putting salt on meat preserve it from spoilage by bacteria? Compare specific transport processes (i.e., diffusion, osmosis, facilitated transport, active transport, endocytosis, and exocytosis) in terms of the following: • concentration gradient • use of channel or carrier protein • use of energy • types or sizes of molecules transported 56 Teacher tip You can provide the learners with key words or allow them to come up with their own key words for their concept map. General Biology 1 Carbohydrates and Lipids: Structures and Functions of Biological Molecules Content Standard The learners demonstrate an understanding of the structures and functions of carbohydrates and lipids and their roles in specific metabolic processes. Performance Standard The learners shall be able to explain the role and significance of carbohydrates and lipids in biological systems. Learning Competencies The learners: • • • categorize the biological molecule as a carbohydrate or lipid according to their structure and function (STEM_BIO11/12-Ii-j-15) explain the role of each biological molecule in specific metabolic processes (STEM_BIO11/12-Ii-j-16) detect the presence of carbohydrates and lipids in food products using simple tests Specific Learning Outcomes At the end of the lesson, the learners shall be able to: • • 120 MINS LESSON OUTLINE Introduction Presentation of learning objectives and important terms; Discussion on dehydration reactions and hydrolysis Motivation 10 Relating the lessons to real-life situations; Discussion on food as sources of energy and building blocks 10 Instruction/ Discussion, as a class and among groups, on Delivery/ the structure and importance of Practice carbohydrates and lipids. 60 Enrichment Laboratory activity on testing the 20 Evaluation 20 presence of carbohydrates and lipids on common food products Group activity on making molecular models of carbohydrates and lipids Materials projector, laptop (if available), sample food labels, common food or drink products (e.g. flour, cornstarch, cooking oil, present simple molecular models of carbohydrates and lipids and relate the food or drink brought by the learners structure to the roles that these molecules play in biological systems perform tests for the presence of starch and reducing sugars and lipids on Resources common food products (1) Reece, J.U. (2011). Campbell Biology, 9th ed. San Francisco, CA: Pearson Benjamin Cummings INTRODUCTION (10 MINS) Teacher tip Communicate learning objectives and important terms Prominently display the learning objectives and important terms on one side of the classroom and frequently refer to them during the discussion. You may place a check-mark beside a term in the wordlist after defining it so that the learners have an idea of their progress. Introduce the following learning objectives using any of the suggested protocols (i.e., verbatim, own words, or read-aloud) • • • I can distinguish a carbohydrate from a lipid given its chemical structure and function. I can explain the roles played by carbohydrates and lipids in biological systems. I can detect the presence of carbohydrates and lipids in food products using simple chemical tests. Introduce the list of important terms that learners will encounter in this lesson: • macromolecule • cellulose • polymer • chitin • monomer • lipids • dehydration reaction • fat • hydrolysis • fatty acid • carbohydrates • triacylglycerol • monosaccharides • saturated fatty acid • disaccharides • unsaturated fatty acid • glycosidic linkage • trans fat • polysaccharide • phospholipids • starch • steroids • glycogen • cholesterol MOTIVATION (10 MINS) 1. Divide the class into groups of three. 2. Distribute sample food or nutrition labels to each group and ask them if they know how to interpret the information written on the food labels. 58 Each learner can also illustrate or define the term on a sheet of paper which can be tacked beside the list of words. Another way of incorporating lists of important terms is to have the words placed in a blank bingo card grid. Learners can write a short definition or description of the term under the entry in the bingo card to block out a square. This may serve as the learners’ reference guide or method of formative assessment. You may ask the following questions to facilitate the discussion and call on several groups to present in front of the class: •How many servings are in this container? •Would you agree that this is the reasonable amount of food you would consume per serving? How many total food calories (C) are in this container? •How much fat is present in one serving? What kind of fat? What is the importance of consuming fats in our diet? •How much carbohydrates are present in one serving? What kind of carbohydrates? What is the importance of consuming carbohydrates in our diet? •Decide on whether this food sample can be eaten often or sparingly and justify. 3.Recall that human beings, like all animals, are heterotrophs that need to take in energy and organic molecules (carbohydrates, fats, and proteins) from plant and animal matter. 4.Explain to the learners that this lesson will describe the structure of carbohydrates and lipids and explain the role that these biomolecules play in important biological processes. Teacher tip For the food labels, local products that are familiar to the learners will make the best samples. Make sure that the labels have carbohydrates, fats, and fibers in them. If there are no food labels available, you may do an image search and print some sample food labels from the internet. Division into small groups of two or three may facilitate sharing. Only call on two or three groups to present if there is limited time. Expect the responses to vary depending on how realistic the serving sizes are. You can also discuss about how advertisers can influence how people perceive food. Take note that a food calorie is the same as 1 kcal or 1000 calories. A young adult would often need to take 1800-2500C per day depending on their size and level of activity. Responses may include saturated, unsaturated, and trans fats. Explain to the learners that these fats will be discussed in more detail during the lesson. Regarding its importance, expect responses ranging from energy source, insulation, for flavor, for aid in cooking, for heart health, skin health, etc. Possible responses include sugar, fibers, etc. Regarding its importance, responses may include energy source, for aid in regular bowel movement, for provision of building blocks for biosynthesis, etc. INSTRUCTION/DELIVERY (60 MINS) Present a diagram similar to the one below. Table 1: Abundant elements in the human body (Source: http://www.personal.psu.edu/staff/m/b/ mbt102/bisci4online/chemistry/elementsorgnsm.jpg) Point out that the bulk (i.e., more than 90%) of the human body weight is provided by only three elements: oxygen, carbon, and hydrogen. We get these elements primarily from the food we eat, from the water we drink, and from the air we inhale around us. Explain to the learners that biogeochemical cycles such as the carbon-oxygen cycle and the water cycle play important roles in ensuring that we have access to these important elements. All forms of life, not only that of humans, are made up of four kinds of important large molecules: carbohydrates, lipids, 60 proteins, and nucleic acids. All of these have carbon atoms as their backbones since carbon is capable of forming up to four chemical bonds with atoms of other elements. Facilitate the lecture on carbohydrates and lipids. What do humans get from food? Heterotrophs, such as human beings, obtain energy and raw materials from food. These are important for cell growth, cell division, metabolism, repair, and maintenance of the body. Nutrients can be classified as either organic nutrients (i.e., those that contain carbon such as carbohydrates, fats, proteins, vitamins, and nucleic acids) or inorganic nutrients (i.e., those that do not contain carbon such as water and mineral salts). What are carbohydrates? Carbohydrates are organic compounds made up of carbon, hydrogen, and oxygen. These compounds have a general formula of CnH2mOm. This means that the hydrogen and oxygen atoms are present in a ratio of 2:1. For example, glucose has a formula of C6H12O6 and sucrose has a formula of C12H22O11. Carbohydrates are usually good sources of raw materials for other organic molecules and energy. One gram of carbohydrates provides four food calories or 16 kJ of energy. In the human diet, carbohydrates mainly come from plants although they are found in all organisms. How are carbohydrates formed? Carbohydrates are examples of macromolecules. These are chainlike molecules called polymers (mere means part) made from repeating units like monomers. Polymers can be formed from covalently-bonded monomers much like a single structure can be made out of repeated building blocks linked to each other. These monomers, called monosaccharides, form covalent bonds when one monomer loses a hydroxyl group and the other loses a hydrogen atom in dehydration or condensation reactions, forming disaccharides. This reaction requires energy to occur. The bond formed is called a glycosidic linkage. Teacher tip Figure 2: Dehydration synthesis of disaccharides from monosaccharide components (Source: https:// bealbio.wikispaces.com/file/view/disaccharides.JPG/364413582/disaccharides.JPG) Longer polysaccharide chains are formed by monomer addition through succeeding dehydration reactions. These reactions can occur in the human liver as carbohydrates are stored as polysaccharides called glycogen or in ground tissues of plants where these are stored as starch. Polysaccharides are broken down into simpler components through the use of water to break covalent bonds and release energy. The process, known as hydrolysis (hydro means water and lysis means split), is the opposite of dehydration reactions and often occurs in the digestive tract during chemical and mechanical digestion. Here, enzymes break bonds within polysaccharides. With the aid of water, one – H group attaches to a monosaccharide while another –OH group attaches to the other. Comprehension question: How many molecules of water are needed to completely hydrolyze a polysaccharide that is one thousand monosaccharides long? 62 Use ball and stick models or plastic blocks to demonstrate how dehydration and hydrolysis reactions occur. Simple reusable ones may be constructed from toothpicks or clay or similar materials. If a projector is available, you may also use animations like the ones found at <http:// www.cengage.com/biology/ discipline_content/animations/ reaction_types.swfto> to help in visualization. Correct response: 999 water molecules During the discussion, invite the learners to find different kinds of carbohydrates in their food labels. How are carbohydrates classified? Carbohydrates can be classified into three main categories, according to increasing complexity: • monosaccharides (monos means single and sacchar means sugar) • • disaccharides (di means two) polysaccharides (poly means many) Some notes on their structures and functions are found in the following table: Classification Monosaccharide Functions • • major cellular nutrient often incorporated into more complex carbohydrates Structure • • • contains a carbonyl group • (C=O) and may be classified as an aldose or ketose depending on the • position may have three to seven carbons in the skeleton may be arranged in a linear form when solid and is converted into a • ring form in aqueous solution (α form when H is on top of plane of ring and β form when -OH is on top of plane of ring) Examples Ribose—a 5C aldose that forms part of the backbone of nucleic acids Glucose—a 6C aldose that is the product of photosynthesis and the substrate for respiration that provides energy for cellular activities Fructose—a 6C ketose that is found in many plants and is often bonded to glucose Classification Disaccharide Functions • • energy source sweetener and dietary component Structure • forms when a glycosidic linkage forms between two monosaccharides Examples • • • Polysaccharide • storage material for important monosaccharides • structural material for the cell or the entire organism • forms when hundreds to thousands of monosaccharides are joined by glycosidic linkages • • Maltose (glucose + glucose)—malt sugar often found in sprouting grains, malt-based energy drinks, or beer Lactose (glucose + galactose)—milk sugar that is a source of energy for infants; an enzyme called lactase is required to digest this. Many adult Filipinos have low levels of this enzyme leading to a condition called lactose intolerance. Sucrose (glucose + fructose)—found in table sugar processed from sugar cane, sweet fruits, and storage roots like carrots Storage polysaccharides are large molecules retained in the cell and are insoluble in water (formed from α 1,4 linkage monomers; with a helical structure) o Starch—amylase is unbranched starch forming a helical structure while amylopectin is branched starch, these are present in plant parts like potato tubers, corn, and rice and serve as major sources of energy. o Glycogen—found in animals and fungi; often found in liver cells and muscle cells Structural polysaccharides (formed from β 1,4 linkage of monomers; strands associate to form a sheet-like structure) o Cellulose—tough sheet-like structures that make up plant and algal cell walls that may be processed to form paper and paper-based products; humans lack the enzymes to digest β 1,4 linkages so is passed out of the digestive tract and aids in regular bowel movement o Chitin—used for structural support in the walls of fungi and in external skeletons of arthropods o Peptidoglycan—used for structural support in bacterial cell walls Teacher tip Examples of alpha helices and beta sheets may be created using wire for the backbone and yarn for the Hbonds; invite learners to speculate on why alpha helix structures are associated with storage polysaccharides and beta sheets with structural polysaccharides. Teacher tip Invite learners to compare the rigidity or structural integrity of plant matter or paper, a shrimp’s shell, and a mushroom. Explain that all these structures are formed from β sheets. What are lipids? Teacher tip Lipids are a class of large biomolecules that are not formed through polymerization. They have diverse structures but are all non-polar and mix poorly, if at all, with water. They may have some oxygen atoms in their structure but the bulk is composed of abundant nonpolar C-H bonds. They function for energy storage, providing nine food calories or 37 kJ of energy per gram. They also function for the cushioning of vital organs and for insulation. Furthermore, they play important roles in plasma membrane structure and serve as precursors for important reproductive hormones. Fats or triacylglycerol formation may be explained better using a diagram such as the one below or through models patterned after a similar diagram. You may ask the learners to explain, in their own words, what they think is happening and compare the formation of carbohydrates with that of lipids. How are lipids classified? Lipids can be divided into three main classes according to differences in structure and function. Some notes on their structures and functions are found in the following table: Classification Functions Fats (triacylglycerols or triglycerides) • energy storage • cushioning of vital organs (adipose tissue) • insulation Structure • • formed from dehydration reactions between glycerol (an alcohol with three Cs, each with an –OH group) forming three ester linkages with three fatty acids (16-18 Cs, with the last C as part of a –COOH group) and producing three molecules of water component fatty acids (FA) may be either saturated or unsaturated o Saturated FA (e.g., palmitic acid) have the maximum number of hydrogen atoms bonded to each carbon (saturated with hydrogen); there are no double bonds between carbon atoms o Unsaturated FA (e.g., oleic acid) have at least one double bond, H atoms are arranged around the double bond in a cis configuration (same side) resulting in a bend in the structure Teacher tip Demonstrate the effects of the straight chains of saturated FAs on packing by piling together flat structures like books or blackboard erasers and ask learners to Examples compare this with the stacking or packing of • Saturated fat—animal products such as irregularly shaped objects like partiallybutter and lard have a lot of saturated fatty acids. folded sheets of cardboard. The linear structure allows for the close packing of the fat molecules for ming solids at room temperature, diets high in these fats may increase the risk of developing atherosclerosis, a condition in which fatty deposits develop within the walls of blood vessels, increasing the incidence of cardiovascular disease • Unsaturated fat—plant and fish oils have unsaturated fatty acids. The bent structure prevents close packing and results in oils or fats that are liquid at room temperature. Homemade peanut butter has oils that separate out of solution for this reason. Industries have developed a process called hydrogenation that converts unsaturated fats into saturated fats to improve texture spreadability. • Trans fat—may be produced artificially through the process of hydrogenation described above. The cis double bonds are converted to trans double bonds (H atoms on opposite sides) resulting in fats that behave like saturated fats. Studies show that trans fat are even more dangerous to health than saturated fats to the extent that they have been banned from restaurants in some countries. During discussion, invite the learners to find different kinds of fats in their food labels and decide on whether a particular food is healthier than another based on its fat content. Misconception Clarify the misconception that consuming fats is entirely dangerous for health. Fats are an essential part of a healthy diet when consumed in moderation. Classification Phospholipids Functions major component of cell membranes • • Structure formed from dehydration reactions between glycerol (an alcohol with three Cs, each with a –OH group), forming two ester linkages with two fatty acids (16-18 Cs, with the last C as part of a –COOH group) and a last linkage with a phosphate group Examples Phospholipids self-assemble into bilayers when surrounded by water and form the characteristic structure of plasma membranes Steroid structure may be explained better using a diagram such as the one below or through models patterned after a similar diagram. You may ask the learners to describe the diagram in their own words and compare the structure of cholesterol with that of other lipids. • Steroids and sterols • regulate fluidity of cell membranes • base of sex hormones • emulsification of fats during digestion • functional group attached to the rings vary (if – OH is attached to the 4th C, then it is called a cholesterol) 66 Phospholipid structure may be explained better using a diagram such as the one below or through models patterned after a similar diagram. You may ask the learners to describe the diagram in their own words and compare the structure of fats with that of phospholipids. Teacher tip phosphate group is hydrophilic and is called the ‘head’ of the molecule fatty acids are hydrophobic and form the ‘tails’ of the molecule • characterized by a Cskeleton with four fused rings Teacher tip • Cholesterol found in cell membranes regulates the rigidity of the cell membrane and are the base material for the production of sex hormones like estradiol and progesterone ENRICHMENT (20 MINS) Divide the class into groups. Instruct the learners to prepare the following materials that are needed for the laboratory activity: • eight glass droppers, medicine droppers, or caps • ethanol solution • 12 test tubes • glucose solution • test tube holders or tongs • flour or cornstarch • beaker • cooking oil • alcohol lamp • sample of studentbrought food or drink • Benedict’s solution • iodine solution • mortar and pestle Explain the following processes to the learners. Benedict’s solution, a blue solution with CuSO4(aq), can detect the presence of reducing sugars (i.e., any sugar with a free aldehyde or ketone group such as all monosaccharides and the disaccharides lactose and maltose). When boiled, these sugars reduce Cu2+ in Benedict’s solution to produce a brickred precipitate of Cu2O(s). Iodine test can be used to detect the presence of starch. Teacher tip This activity may be done as a class if time does not permit for the activity to be done in separate groups. If Benedict’s solution is not available, you may only perform the last two tests. In the absence of laboratory grade chemicals, you may improvise with storebought chemicals like iodine and 70% ethyl alcohol for medical use. Make sure to test the procedure before performing the activity in the class. Emulsion test can be used to identify fats. Learners should perform all three tests on the following samples: • glucose solution (available in the baking section of grocery stores) • cooking oil food or drink sample that the learners • flour or cornstarch solution brought. For solid samples, instruct the learners to mash a small portion of the sample in some water using the mortar and pestle and then test the resulting solution. Ask the learners to prepare a table with appropriate headings in which to record their results. • In discussing the results, ask the learners to conclude whether carbohydrates or lipids are present in their samples. They may compare this with the list of ingredients for their food or drink sample. They can also list possible sources of errors. EVALUATION (20 MINS) Divide the class into small groups. Provide the groups with different structures of lipids or carbohydrates and ask them to create models using common or recyclable materials. Ask the learners to explain or write a short description of their models. In grading the models, check to see if the learners were able to create an accurate model of the assigned lipid or carbohydrate. Ask the learners, still in their small groups, to create a short flowchart that will allow them to distinguish between the different kinds of carbohydrates and lipids based on their structures. They may use this flowchart in answering the comprehension questions that follow. Provide different molecular structures of the following and ask the learners to identify whether these are: 68 Teacher tip Prior to this lesson, instruct the learners to bring recyclable materials that they can use for this activity. • • monosaccharides disaccharides • unsaturated fats • storage polysaccharides • phospholipids • structural polysaccharides • steroids. • saturated fats You may also ask the learners to give one of the associated functions or characteristics of the given carbohydrate or lipid. Teacher tip The various carbohydrate structures were obtained from the following electronic resources: • • • commons.wikimedia.org http://www.nature.com/pj/journal/v43/ n12/images/pj201196f3.jpg http://chemwiki.ucdavis.edu/@api/deki/ files/522/260px-Cellulose_strand.jpg? size=bestfit&width=352&height=310&r evision=1 Images for the various lipid structures were obtained from the following electronic resources: • https://upload.wikimedia.org, • http://www.mikeblaber.org/oldwine/ BCH4053/Lecture13/triglyceride.jpg, https://my.bpcc.edu/content/blgy225/ Biomolecules/phospholipid.gif General Biology 1 180 MINS Amino Acids and Proteins Pt. 1 of 2 Content Standard The learners demonstrate an understanding of the structure and function of biomolecules (i.e., proteins). LESSON OUTLINE Introduction Review on the Genetic Code; Translation 20 Motivation 10 of codons to corresponding amino acids Class activity on the Protein or Amino Acid Alphabet Performance Standard Instruction/ Lecture-discussion on the different levels The learners shall be able to construct a three-dimensional model of proteins Delivery of protein structure (i.e., primary, using computers (i.e., computer generated models) or recyclable materials (i.e., secondary, tertiary, and quaternary physical models). Practice Learning Competencies The learners: • • • categorize the biological molecule (i.e., protein) according to their structure and function (STEM_BIO11/12-Ii-j-15) explain the role of each biological molecule in specific metabolic processes (STEM_BIO11/12-Ii-j-16) determine how factors such as pH, temperature, and substrate affect enzyme or protein activity (STEM_BIO11/12-Ii-j-19) Specific Learning Outcomes At the end of the lesson, the learners shall be able to: • • • Class activity on paper models of the different protein helix types 15 Evaluation 60 generated protein models; Identification of surface features of proteins (e.g., hydrophobic patches, positively or negatively charged domains, etc.) Examination and Model Accuracy Evaluation recyclable materials for construction of protein models, software for molecular modelling (available for free download) Resources (1) SwissPDB Viewer software (available for free download) (2) Protein Data Bank (can be accessed at www.db.org 70 15 Enrichment Constructing of physical or computer- Materials discuss the different levels of protein structure (i.e., primary, secondary, tertiary, and quaternary) discuss how protein structural features may influence their interactions discuss how protein structural features may influence their functions 60 INTRODUCTION (20 MINS) Teacher tip Communicate learning objectives and important terms 1. Review the Genetic Code. In particular, have the learners translate codons to their corresponding amino acids. 2. Stress the importance of how mutations may alter the type of amino acid coded by a given sequence. 3. Discuss how some mutations may be ‘silent’. 4. Discuss how some mutations may be considered ‘conservative’ (e.g., AA changed to another with a similar functional group type) or ‘non-conservative’ (e.g., AA changed to another with a different functional group type). 5. Discuss the importance of the translation reading frame. Stress how frameshift mutations may lead to changes in the amino acid sequence translation as well as changes in the termination of the protein. Prepare a genetic code table. Teach the learners how to use the genetic code table to translate an mRNA sequence. MOTIVATION (10 MINS) 1. Instruct the learners to spell out their names using the amino acid single letter codes (e.g., NEIL). 2. Next, instruct them to spell out their names using the amino acid triple letter codes (e.g., Asn-GluIle-Leu). 3. Then have the learners find codons that can correspond to these amino acid sequences. INSTRUCTION/DELIVERY (60 MINS) 1. Discuss the basic amino acid structure. Discuss how these amino acids may be linked by peptide bonds to form polypeptides sequences. 2. Group the amino acids in terms of their functional group properties. The amino acids may be polar (i.e., acidic or basic) or non-polar. 3. Highlight amino acids with special properties (e.g., Cysteine for disulfide bond formation; Tyr, Trp, and Phe for their aromatic rings and fluorescent character; Proline for its effect on the protein backbone). 4. Discuss how the amino acid sequence is the primary structure of the protein. 5. Discuss how properties in the primary structure (e.g., placement of hydrophobic residues, charged groups, and disulfide bridges) define the higher level structures of the protein. Show how interactions between complementary sections of polypeptide chains may lead to the formation of helices or sheets. Clarify the misconception on mutations in the mRNA sequence. Stress how not all mutations in the mRNA sequence may lead to changes in amino acid sequence. Teacher tip Prepare an Amino Acid Alphabet table. In the table, provide respective columns for one-letter and three-letter codes for each amino acid. Present the Genetic Code table with the Amino Acid Alphabet table so that the learners can use them in the translations. 6. Discuss the common secondary structures of proteins (i.e., helical and sheet-like structures). 7. Discuss the different helical types (3-10 helices, alpha helices, and pi helices). Show how these different types are based on which residues are bound by hydrogen bonds or how many atoms are included in the helices hydrogen bonding network. 8. Show how some proteins are composed of combinations of the secondary structures in differing ratios. 9. Discuss how the arrangement of the secondary structures in these proteins may lead to the formation of functional regions (e.g., active sites). 10. Discuss how protein functions may involve the interaction of several proteins in what is known as quaternary associations (e.g., protein complexes for signal transduction). PRACTICE (15 MINS) Teacher tip Note that signal transduction pathways commonly involve quaternary protein structures. The maintenance of proper interactions in these structures is necessary to produce the desired functions. Dysfunction in these associations is commonly associated with disease. Some current cancer diagnostic kits target mutations that may disrupt the proper association of proteins in these complexes. Instruct the learners to construct paper models of helical structures. Paper models may be made to represent structures such as the 3-10 helix and the alpha-helix. This would require the drawing of polypeptide chains on paper, and the folding of the paper ensuring that the peptide bonds are kept planar. The linkage of the appropriate amide protons (NH) with the appropriate C=O group will create models of the proper dimensions (i.e., angle and width). This is based on the classic experiment by Linus Pauling on the discovery of the alpha helix. ENRICHMENT (15 MINS) Prior to this lesson, the learners may generate computerized protein models and bring them to class. Also, software on molecular viewers should be downloaded from the internet. These software, such as the SwissPDB Viewer, can be downloaded for free. Sample protein structures may be downloaded from the Protein Data Bank that can be accessed at www.pdb.org. Determine the surface features of the observed protein (e.g., hydrophobic areas, charged areas). If a protein with an active site and a bound ligand is chosen (eg., PDBID _____),then the location of the active site and the nature of the protein-ligand interaction may be explored. EVALUATION (60 MINS) Conduct an examination to assess the learners’ knowledge and understanding of the discussions. 72 Teacher tip A video on the discovery of the alpha helix using paper models is available on the internet. This video features Dr. Linus Pauling himself describing the general steps in creating an alpha helix paper model. Teacher tip Familiarize yourself with the features of the molecular viewer. SwissPDB Viewer allows you to select amino acids of certain types (e.g., hydrophobic residues). Teacher tip These may be colored or labelled to show their positions in the protein. The position of hydrophobic patches and charged surfaces in proteins can signify areas of potential interaction. General Biology 1 Amino Acids and Proteins Pt. 2 of 2 Content Standard The learners demonstrate an understanding of the structures and functions of biological molecules (i.e., carbohydrates, lipids, nucleic acids, and proteins) Performance Standard The learners shall be able to identify key structural features of biological molecules that are important for their functions (e.g., 5’ and 3’ OH of DNA, 2’ OH of RNA, complementary base pairing, N and C termini of proteins, R groups of the different amino acids, etc.). Learning Competencies The learners: • • • • categorize the biological molecules (e.g., DNA, RNA, proteins) according to their structure and function (STEM_BIO11/12- II-j-15) explain the role of each biological molecule in specific metabolic processes (STEM_BIO11/12 -II-j-16) explain oxidation/reduction reactions (STEM_BIO11/12- II-j-18) determine how factors such as pH, temperature, and substrate affect enzyme or protein activity (STEM_BIO11/12 -II-j-19) Specific Learning Outcomes At the end of the lesson, the learners shall be able to: • • • • discuss key structural features of DNA, RNA, and proteins discuss structural and functional differences between DNA and RNA discuss the different levels of protein structure (i.e., primary, secondary, tertiary, and quaternary) discuss how protein structural features may influence their functions 60 MINS LESSON OUTLINE Introduction Review on Cell and its organelles; Discussion 5 and illustrations of the Central Dogma of Molecular Biology Motivation 5 Class activity on the important functions of biological molecules` Instruction/ Lecture-discussion on the main functions and Delivery important physical properties of 30 biomolecules Practice 5 Exercise on translating coding to non-coding sequences Enrichment Practice exercise on translating coding 5 sequences into mRNA transcripts and mRNA transcripts into polypeptide sequences. Evaluation Practice exercises on identification of biomolecules based on given chain structures, identification of important structural features in the chain structures, and generating non-coding sequences (DNA), transcripts (RNA) and polypeptides to assess learners’ understanding of the topics 10 Materials recyclable materials for construction of models of biological molecules, software for molecular modelling (available for free download) Resources (1) SwissPDB Viewer software (available for free download) (2) Protein Data Bank (can be accessed at www.db.org INTRODUCTION (5 MINS) Teacher tip 1. Facilitate a review on the cell and its organelles. Emphasize that the specific functions for each organelle type are compartmentalized and that the functions of each organelle are defined by its physical properties. 2. Explain to the learners that the lesson will focus on understanding the important physical properties of certain biomolecules (i.e., DNA, RNA and proteins) and how these properties allow the biomolecules to serve specific functions within the cell. RNA Protein 3. Discuss the Central Dogma of Molecular Biology: DNA Relate the Central Dogma of Molecular Biology to real world situations. For example, in cooking, the cook book functions like the DNA (Genome). The specific recipe functions like the mRNA while the desired dish is the protein. Provide two more examples of everyday life activities that can illustrate the Central Dogma of Molecular Biology. Teacher tip MOTIVATION (5 MINS) 1. Divide the class into groups and instruct them to identify or enumerate the most important functions of DNA, RNA, and proteins. 2. Consolidate the learners’ responses on the board. Elaborate on the main functions of the biomolecules: • DNA—is the repository of genetic information RNA—serve as the transcripts and regulators of expressed genetic information Proteins—are the functional products and executors of cellular functions Biomolecule DNA Physical Property • • INSTRUCTION/DELIVERY (30 MINS) • • • Note the following expected responses: Functional Relevance Complementary Base Pairs Allows each strand to serve as a template for replication and transcription Phosphodiester bonds Essential for polynucleotide chain elongation Deoxyribose 5’OH Start of the polynucleotide chain Deoxyribose 3’OH “End” of the polynucleotide chain Connection point for extending the chain 74 DNA—repository of genetic information RNA—transcripts and regulators of expressed genetic information protein—functional products and executors of cellular functions Biomolecule RNA Protein Physical Property Functional Relevance Teacher tip Use computer modelling software like Deoxyribose 2’H Difference between the sugar residues of DNA (deoxyribose) SwissPDB Viewer to illustrate the basic structures of DNA, RNA, and Proteins and RNA (ribose) Complementary Base Pairing Allows RNA to serve as transcripts (mRNA) and translators (tRNA) of genetic information from DNA. Uracil Nitrogenous base equivalent to T in RNA. Ribose 2’OH Difference between the sugar residues of DNA (deoxyribose) and RNA (ribose) Focus on the important parts of the structure that provide the necessary physical Limits the compaction of RNA molecules. properties of DNA, RNA, and Proteins. Double stranded RNA molecules are similar in structure as the A-form of DNA N-Terminus Start of the polypeptide chain C-Terminus End of the polypeptide chain Addition point for new amino acids during polypeptide growth Peptide Bond Links Amino Acids Planar character Phi Angle Angle between: Ci-1-Ni-Cαi-Ci Ci-1 : Carbonyl C of previous AA Ni : Amide Nitrogen of current AA Cαi: Alpha Carbon of current AA Ci : Carbonyl C of current AA Angle is observed by looking down the bond between Ni and Cαi; coming from the N-terminus of the polypeptide (Polypeptides). The basic structures for these biomolecules are available as molecular structure files (*.pdb) from the Protein Data Bank that can be accessed at www.pdb.org. Discuss the relevance of these physical features for the functions of DNA, RNA, and Proteins. Biomolecule Physical Property Psi Angle Functional Relevance Angle between Ni+1-Ci-Cαi-Ni Ni+1 : Amide Nitrogen of succeeding AA Ci : Carbonyl C of current AA Cαi: Alpha Carbon of current AA Ni : Amide Nitrogen of current AA Angle is observed by looking down the bond between Ci and Cαi; coming from the C-terminus of the polypeptide Defines Amino Acid Character Amino Acid R-Groups a. non-polar i. aliphatic (G,A,V, L, I, M) ii. aromatic (Y,W,F) b. polar, uncharged (S,T,C,P,Q) Table 1: Important Physical Properties of Biomolecules Teacher Tip: PRACTICE (5 MINS) Complementary Non-coding/ sequence: 3’ TACGTATCTAATCCTATAGGGTCTATC 5’ The correct response is: Given the following coding sequence for DNA, provide the sequence of the complementary (template) sequence. Coding sequence: 5’ ATGCATAGATTAGGATATCCCAGATAG 3’ 76 Be sure to note the antiparallel orientation of the coding and non-coding strands of the DNA. Note the relative positions of the 5’ and 3’ ends. ENRICHMENT (5 MINS) Convert the given coding sequence into an mRNA transcript: Complementary Non-coding / Template sequence: 3’ TACGTATCTAATCCTATAGGGTCTATC 5’ 2. Translate the given mRNA transcript into a polypeptide sequence: Coding sequence ~ mRNA transcript: 5’ AUGCAUAGAUUAGGAUAUCCCAGAUAG 3’ Teacher tip The correct responses are the following: For no. 1, the coding sequence ~ mRNA transcript is 5’ AUGCAUAGAUUAGGAUAUCCCAGAUAG 3’ For no. 2, the polypeptide sequence is N-Met-His-Arg-Leu-Gly-Tyr-Pro-Arg-C Note that the mRNA transcript has almost the same sequence as the coding sequence (DNA), but the Thymines are converted to Uracil. Teach the learners how to read the Codon Table. Teach the learners the single letter codes for the amino acids (e.g., Tryptophan Trp W). EVALUATION (10 MINS) Ask the learners to identify the type of biomolecule represented by a given chain structure: • DNA • RNA • Protein You may ask the learners to identify the important structural features in these chain structures. The features are listed in Table 1 in the Instruction/ Delivery section of this Teaching Guide. A similar exercise of generating non-coding sequences (DNA), transcripts (RNA), and translated polypeptides may be performed to test the learners’ understanding of the topic. Instruct the learners to spell their names using the amino acid codes (e.g., N-E-I-L Asn – Glu – Ile – Lue). Teacher tip Worksheets with partially-completed sequences may be used to help the learners practice the generation of complementary sequences. For example: Template sequence 3’ TAC_ _ _TCT_ _ _ CCTATAGGGTCT 5’ 5’ _ _ _CAUAGAUUA_ _ _UAU_ _ _AGA 3’ General Biology 1 150 MINS Biological Molecules: Enzymes LESSON OUTLINE Introduction Presentation of objectives and terms; 5 Content Standard The learners demonstrate an understanding of enzymes and of the factors affecting enzyme activity. Motivation 5 Performance Standard The learners shall be able to explain the role and significance of enzymes in biological systems. Instruction/ Small-group and class discussion on Delivery/ definition of enzymes, its structure, and Practice function 60 Learning Competencies The learners: Enrichment Laboratory activity on the work of 60 Evaluation 20 • • describe the components of an enzyme (STEM_BIO11/12-Ii-j-17) determine how factors such as pH, temperature, and substrate affect enzyme activity (STEM_BIO11/12-Ii-j-19) Brief discussion on thermodynamics or protein structure enzymes using raw liver as a source of catalase and hydrogen peroxide as the substrate Specific Learning Outcomes At the end of the lesson, the learners shall be able to: • • present a model demonstrating the components of a specific enzyme in a biological system and the reaction it catalyzes design a simple experiment that illustrates how pH, temperature, or amount of substrate affect enzyme activity (i.e., includes problem statement, hypothesis, materials and methods) 78 Illustration and explanation of enzymatic browning in bananas Group activities on creating models of enzyme-catalyzed reactions and designing of a simple experiment on enzyme activity Materials projector, computer, recyclable materials for making models of enzyme-catalyzed reactions. Resources (1) Reece, J.U. (2011). Csmpbell Biology, 9th ed. San Francisco, CA: Pearson Benjamin Cummings. INTRODUCTION (5 MINS) Introduce the following learning objectives using any of the suggested protocols (e.g., verbatim, own words, or read-aloud): • • I can describe the components of an enzyme. I can determine how factors such as pH, temperature, and substrate affect enzyme activity. Introduce the list of important terms that the learners will encounter: • • • • • • • • • • • enzyme catalyst activation energy substrate enzyme-substrate complex active site induced fit cofactor coenzyme competitive inhibitor noncompetitive inhibitor MOTIVATION (5 MINS) Connect the lesson to a real-life problem or question. While the learners are coming in and settling down in the classroom, show them that you are cutting a banana into small pieces on a plate. After the introduction, you can show the learners the plate of banana pieces. Instruct them to pass it around and share their observations. The following could be expected answers: • • • • • The banana’s covering is brown. The banana’s texture is mushy. The banana looks spoiled. The banana is smelly The banana is soft. Teacher Tip: Prominently display the learning objectives and important terms prominently on one side of the classroom and frequently refer to them during discussion. You may place a check-mark beside a term in the wordlist after defining it so that the learners have an idea of their progress. Another way of incorporating lists of important terms is to have the words placed in a blank bingo card grid. Learners can write a short definition or description of the term under the entry in the bingo card to block out a square. This may serve as the learners’ reference guide or method of formative assessment. Teacher Tip: You may opt to divide the class into groups of twos or threes to facilitate sharing of observations and insights. You may call on two to three groups to share their observations if there is limited time for presentations. Ask the learners if they can explain why the bananas have turned brown and mushy. Challenge the learners to think of ways to slow down the process of browning. Expected answers could be not to cut the banana, keep the banana cold, or keep the banana in an airtight container. Provide the learners with the following explanation: • • • Peeling, bruising, or cutting fruits cause them to release enzymes like polyphenol oxidase (PPO, phenolase) that, with the presence of oxygen in the surrounding air, goes into chemical reactions of plant compounds. These chemical reactions produce brown pigments through the process of enzymatic browning. Enzymes—are organic substances that accelerate the rate of chemical reaction. Enzymatic browning can be a significant problem because it limits the shelf life of fruits and vegetables. However, enzymatic browning is not always unwanted. The browning reaction contributes to the desirable color and flavor of raisins, prunes, coffee, tea, and cocoa. Although enzymatic browning causes changes in flavor and taste (i.e., bitter, astringent) and may reduce quality, the browning agents formed are not toxic. Brown fruits are safe to eat up to a few hours after cutting. Knowing the mechanism behind this, Arctic Apples, a Canadian company, produced geneticallyengineered apples that will not brown for 15-18 days. INSTRUCTION/DELIVERY/PRACTICE (60 MINS) Recall previous lessons on the laws of thermodynamics, spontaneity of reactions, and carbohydrate structure through a scenario. • • • Scenario: Is the hydrolysis of starch to maltose a spontaneous reaction? What information do you need to answer this question? Expected response: Question may be answered with the given information since we are familiar with hydrolysis and the structures of starch and maltose. The reactants have higher levels of free energy compared to the products, making this a spontaneous reaction. Follow-up questions: If this is so, why will a sterile starch solution sit for years at room temperature without significantly hydrolyzing? Can you think of ways to hydrolyze starch more quickly? 80 Teacher Tip: If pressed for time, No. 1 can be done as part of the Motivation with soda crackers or plain biscuits (that is mostly composed of starch) as an example. Moisten the cracker with water and place it in a visible area. Compare the time it takes for a similarlysized piece of cracker to break down inside a learner’s mouth. Remind the learner not to chew the cracker. If available, amylase and Benedict’s solution may be used to test for the presence of simple sugars. Numerous chemical reactions support life. The regulation of these reactions may take place in two major ways: • through the use of enzymes • through the regulation of genetic material (This which will be discussed later.) Proceed to the lecture proper. • Can you imagine what would happen if it takes many years for our body to hydrolyze the starch in the food that we eat? Starchy food comprises an important part of our diet and our bodies use enzymes called amylase to quickly hydrolyze starch into simple sugars. • • What are enzymes? Enzymes are organic or biological catalysts. Catalysts are substances that speed up a reaction without being used up, destroyed, or incorporated into the end product. They are vital to the regulation of the metabolic processes of the cell. Many enzymes are proteins. We will focus on this type of enzymes in this discussion. RNA enzymes called ribozymes will be discussed later. • What keeps spontaneous reactions from occurring more rapidly? • All chemical reactions between molecules involve the breaking and forming of bonds. Converting starch into glucose involves contorting starch into a highly unstable state before the reaction can proceed. This unstable state is called the transition state that happens when reactants absorb energy from their surroundings and. This initial investment of energy in order to start a reaction is called the activation energy. It is often supplied as thermal energy or heat absorbed by reactants from their surroundings. Reactant molecules absorb heat which causes them to collide more frequently and more forcefully. This agitates the atoms within the molecules that results in the likely breaking of bonds. • When the new bonds of the products form, energy is released as heat and the molecules return to stable shapes with lower energy. This results in an overall decrease of free energy. Figure 1 (on the right): Energy profile for a spontaneous exergonic reaction: AB+CDAC+BD (Source: Reece, J. U (2011 ). Campbell Biology, 9th ed. . San Francisco, CA: Pearson Benjamin Cummings) Teacher Tip: A visual aid may be used to show the contortion. For example, a string of paper clips or a bunch of keys on a key ring can stand for starch. The contortions involved in detaching one paper clip or removing one key can serve as analogies for the process. Teacher Tip: To illustrate the concept of activation energy, pushing a toy car or marble up a makeshift ramp will also help concretize the concepts of activation energy and transition state. • The reaction shown in Figure 1 is spontaneous but the activation energy provides a barrier that determines the rate of the reaction. Reactants have to absorb enough energy from their environment to surmount this barrier before the reaction can proceed. • Knowing this, how can you cause reactants to absorb more energy from their environment? How do enzymes affect reactions? Heat speeds up reactions. This is inappropriate for biological systems because it denatures proteins, kills cells, and speeds up all reactions, not just those that are needed. Enzymes catalyze specific reactions by lowering the activation energy barrier and allowing the reactant molecules to absorb enough energy at moderate temperatures. Enzymes cannot change the !G for a reaction and can only hasten reactions that would eventually occur anyway. View Part I of the animation at http://www.sumanasinc.com/webcontent/animations/content/enzymes/ enzymes.html. Click on ‘Show Narrative’ to reinforce the aforementioned concepts on spontaneous reactions. Ask the learners to answer the following questions and call on a small group to explain their responses using their own words: • • What is the activation energy of a reaction? How do enzymes affect the activation energy of a reaction? Enzyme structure and function View http://highered.mcgraw-hill.com/sites/0072495855/student_view0/chapter2/ animation__how_enzymes_work.html. Click on ‘Text’. Ask the learners to answer the following question and call on a small group to explain their responses using their own words: On a molecular level, how does the shape of an enzyme enable it to perform its function? Use the following terms in your responses: ‘active site’, ‘substrate’, ‘enzyme-substrate complex’, and ‘product’. The active site and functional groups of its amino acids may lower activation energy by: • • • • acting as a template for substrate orientation stressing the substrates and stabilizing the transition state providing a favorable microenvironment participating directly in the catalytic reaction 82 Teacher tip Relate the discussion with the hypothetical reactions in Figures 1 and 2. View http://www.wiley.com/college/boyer/0470003790/animations/enzyme_binding/ enzyme_binding.htm. Click on ‘Binding Models’. Ask the learners to answer the following questions and call on a small group to explain their responses using their own words: • • How does the shape of an enzyme enable it to perform its function? What is the difference between Emil Fisher’s lock-and-key model and Daniel Koshland’s induced-fit model? Describe the current model. Factors that affect enzyme action Given what the learners know about enzyme action, ask them to predict how the following factors will affect the action of an enzyme by completing the table below. This may be done by group or by the class as a whole. If the entire class is involved, invite a small group to fill in each row. Table 1: Factors that affect enzyme action. Teacher tip The video links provided here may be given as a pre-lecture assignment to stimulate prior knowledge and give the learners an idea of the topics to be discussed. If a computer is not available, you may ask the learners to answer the questions based on your discussion. Provide constructive feedback and correct misconceptions. You may use models or analogies to better illustrate the concepts. Here are some suggestions: • a handshake to demonstrate induced fit • two pieces of a jigsaw puzzle to illustrate components of a substrate • roleplay to show the interactions between enzymes and substrates Clarify the misconception that enzymes only function in catabolic reactions or breaking down of substances. This misconception may be due to the learners’ first encounter of enzymes in the context of digestion. Enzymes also catalyze other types of reactions. For example, DNA polymerase facilitates the addition of nucleotides to a polynucleotide chain. View http://moleculesoflife2010.wikispaces.com/file/view/Enzyme+Model.swf and test the hypotheses using controlled simulations. You may need to use a timer to assess reaction rates. Do your results agree with your predictions? How were they similar? How were they different? Provide constructive feedback and clarify the reasons for the observed changes to reaction rates and enzyme function. View http://web.biosci.utexas.edu/psaxena/MicrobiologyAnimations/Animations/Enzyme-Substrate/micro_enzyme-substrate.swf. Ask the learners to answer the following question and call on a small group to explain their response in their own words: What is the difference between a competitive and noncompetitive inhibitor? The presence of non-protein helpers called co-factors and of organic molecules like co-enzymes may activate apoenzymes to produce holoenzymes by binding to their active sites. Common examples may be found in popular supplements such as ions of iron, copper, zinc, or in vitamins like vitamins A, C, and B-complex. Enzyme regulation and metabolic control View http://usmanscience.com/12bio/enzyme/enzyme_animations.htm. Click on ‘Allosteric Enzymes’ and ‘Feedback Inhibition’. Ask the learners to answer the following questions and call on a small group to explain their responses in their own words: What are allosteric enzymes? Feedback inhibition regulates metabolic pathways that use more than one enzyme. How does feedback inhibition work? ENRICHMENT (60 MINS) Facilitate a laboratory activity on investigating the work of enzymes using raw liver as a source of catalase and hydrogen peroxide as the substrate. Learners may be provided with the following: • • • • temperature as a factor: ice water bath, water bath at room temperature, warm water bath pH as a factor: acid solution, alkaline solution, and litmus paper amount of substrate: droppers small test tubes or medicine caps Explain that many living tissues contain catalase or peroxidase that catalyzes the reaction conversion of H2O2 → H2O + O2. Cut the liver into equal-sized cubes and demonstrate the effect of placing the liver in a 2mL solution of hydrogen peroxide. Evolution of gas (i.e., bubbling) will be observed. Groups of learners may prepare solutions that they can test using the different factors. Instruct the learners to rank the different solutions based on the rates of reactions. 84 Teacher tip This activity may be done as a class if there is not enough time to perform the laboratory work individually or in groups. You may perform the test on all three factors or you may choose only one or two. EVALUATION (20 MINS) Making models of enzyme-catalyzed reactions 1. Divide the class into small groups. 2. Distribute different examples of important enzyme-catalyzed reactions to the groups. 3. Ask the groups to create models using common or recyclable materials and label the following components: enzyme, substrate, product, and active site. 4. Ask the learners to demonstrate how this enzyme works. Written Task 1. Divide the class into small groups. 2. List the materials for Enrichment (See previous section). 3. Ask the learners to design their own experiment in order to explore the effect of one factor on enzyme activity. Explain that the experimental design should include: • • • • • • a testable hypothesis complete list of dependent and independent variables complete list of controlled variables logical procedure in a flowchart format short and accurate explanation of what you expect to happen and why sufficient replicates Teacher tip Before this session, inform the learners that they will be making models of enzymecatalyzed reactions next meeting and ask them bring recyclable materials that they can use for this activity. In grading the models, check to see if the learners were able to label the parts correctly. Demonstrate that the substrate goes into the active site, gets contorted and moves out as a product. Check if the substrate and the products are consistent with the reaction being modeled (e.g., anabolic, catabolic, or recombination) General Biology 1 240 MINS Photosynthesis and Cellular Respiration LESSON OUTLINE Introduction Communicate to the class the oxidation- 5 Content Standard The learners demonstrate an understanding of photosynthesis and cellular respiration Motivation 5 Learning Competency The learners: Instruction/ Show the overall equations of photosynthesis 145 and cellular respiration Delivery • reduction and the flow of energy Describe the major features and chemical events in photosynthesis and cellular respiration (STEM_Bio11/12-IIa-j-1) Specific Learning Outcomes At the end of the lesson, the learners shall be able to: • • • • functionally define photosynthesis and cellular respiration; identify the reactants and products of photosynthesis and cellular respiration; differentiate the major chemical events of photosynthesis and cellular respiration; and summarize in a form of illustration or diagram the similarity in the organization of chloroplast and mitochondrion in carrying out photosynthesis and cellular respiration, respectively. Post questions on the board and ask the students to identify the processes involved in energy transformation Enrichment Similarity of photosynthesis and cellular 25 Evaluation 60 respiration and connecting the concepts with the biological systems Summary of the major events of photosynthesis and cellular respiration Resources (1) Alumaga, Maria Jessica B. et al., (2014). Science and Technology 9. Quezon City: Vibal Publishing House (2) Mader, Sylvia S. (2010). Biology 10th Edition. USA: McGraw-Hill (3) Solomon, Eldra P. et al., (2008). Biology 8th Edition. China: Thomson Brooks/ Cole (4) www.biologycorner.com accessed July 19, 2015 Suggested Media Tools • • • www.mhhe.com/maderbiology11 www.masteringbiology.com www.biologycorner.com For animations, video, learning outcomes, chapter outline, image PowerPoint, essay quiz, thinking critically, practice test and concept maps: • http://highered.mheducation.com/sites/0073403466/student_view0/ chapter7/image_powerpoint.html • http://highered.mheducation.com/sites/0073525502/student_view0/ chapter7/index.html 86 INTRODUCTION (5 MINS) Review with the class that oxidation-reduction (redox) reactions involve electrons passing from one molecule to another. Oxidation (also splitting) is the loss of electrons while reduction is the gain of electrons. You can show this picture to your students and try to ask questions so that you can generate critical-thinking skills from them. To help them visualize the concept, a diagram of redox reactions is also shown below. Ask your students which organisms (in the picture below) photosynthesize and which respire (take note that plants both photosynthesize ad respire at the same time). Then show the equation of redox reactions after your students have given their responses. You may also ask examples of oxidation reactions (e.g., browning of peeled potato, banana, and eggplant). For redox reactions examples are rusting of iron, burning of combustible material (e.g., wood, coal, etc.) NOTE: Energy transformation (e.g., photosynthesis and cellular respiration is one of the difficult topics in biology. To capture the general picture of the topic, students have to be encouraged to read and reread the key concept, write and re-write, outline and re-outline, draw and re-draw, and to recite orally if they want the ideas to sink in their system. Patience and steadfastness are important virtues that should be included as you study this concept. Teacher Tip: Note: This lesson merely describes the major features of (or an overview) photosynthesis and cellular respiration. A more detailed concept and deeper explanation will be presented in another set of learning competencies. Emphasize that the flow of energy starts with the sun. There are two organelles that participate in the energy flow from the sun through living things. You can now motivate your students by posting two questions relating to energy transformation. Redox reactions is one type of chemical reaction. Emphasize to students the importance of understanding the processes rather than memorizing all the various reactions. Remember that plants both photosynthesize and do on aerobic respiration. The following are some of the practical examples of photosynthesis: 1. Photosynthesis helps create food chains or food web (Note: Most modern scientists prefer the latter because it portrays the accurate interactions of several organisms in the environment. The interactions make possible the production and perpetuations of the living creatures. Most life forms directly or indirectly depend on plants for their basic metabolism. Ultimately, materials from producers, herbivores, omnivores and carnivores will be consumed by decomposers (e.g., bacteria). These bacteria produce waste products that increase the nutrient content of the soil. Thus, plants are able to produce macromolecules (e.g., carbohydrates, proteins, fats and nucleic acids) and sustain other cellular activities because of the participation of the soil, nutrients, water, carbon dioxide, chlorophyll and sunlight as preparatory materials for the synthesis of carbohydrates and oxygen. Countless chemical reactions are occurring in cells to do essential life functions with the help of ATP as the energy currency of the cells. Ask your students what are the tasks of ATP. The following are the answers: Photosynthesis and bioenergy. The plant materials and animal wastes are used especially as a source of fuel. 1. Chemical work: ATP is used for building macromolecules 2. Transport work: ATP is used for transporting ions membranes 3. Mechanical work: ATP is used for mechanical processes such as muscle contraction, cilia movement For additional information, tell the class that ATP is also involved in rigor mortis—a temporary stiffness of the body that happens soon after death of a person. Teacher tip MOTIVATION (5 MINS) Post these two questions on the board. Ask them to identify the process involved in each question so that food is manufactured and energy is released. • • How do plants harness light energy to manufacture food? How do living organisms harness energy from food? Then show to them the overall equation for each process as follows: • • A. 1. Components that are utilized: Carbon dioxide, water, sunlight (and chlorophyll can be mentioned) 2. Groups that come out: Carbohydrate (glucose) and oxygen B. 1. Groups that go in: Carbohydrate, oxygen and 38 ADP molecules 2. Groups that are released: Carbon dioxide, water and 38 ATP molecules Chemical reactions for photosynthesis: 6 CO2 + 6 H20 + sunlight Suggested answers: 1. Through photosynthesis 2. Through cellular respiration C6H12O6 + 6 O2 Which groups participate in the reaction? 88 • • Which groups are released? Chemical reactions for cellular respiration: C6H12O6 + 6 O2 + about 38 molecules of ADP • Which groups participate in the reaction? • Which groups are released? 6 CO2 + 6 H20 + about 38 molecules of ATP INSTRUCTION/DELIVERY (145 MINS) 1. You can draw pictures of photosynthesis and cellular respiration in Manila paper if LCD is not available. You can also go to computer/printing shop and make these pictures into tarpaulin for long use. • Sample picture of Overview of Photosynthesis, Overview of the Stages of the Calvin Cycle in Photosynthesis, Overview of Glucose Breakdown, and Overview of ATP Yield per Glucose Molecule may be viewed at Biology 10th Edition by Mader, Sylvia S. (2010) (Retrieved July 20, 2015) 2. Group your students into triad according to their learning skills. Give each member accountability task to promote mutual cooperation. 3. Give them questions to answer for discussions. Tell them to prepare and bring out their Manila paper and markers. 4. Have them report orally to the class. 5. Alternatively, if the class will not be able to report reliably, roleplaying, laboratory activities, or simulations involving computer-aided activities such as the ones found in the following sites: • http://www.reading.ac.uk/virtualexperiments/ves/preloader-photosynthesis-full.html • http://www.pbs.org/wgbh/nova/nature/photosynthesis.html Processing Questions: What are the two kinds of reactions in photosynthesis? What are the basic stages of the Calvin cycle? What are the reactants and products of photosynthesis? In which part of the cell glycolysis happens? What about the citric acid cycle and electron transport chain? 5. How many metabolic pathways are there in cellular aerobic respiration? In anaerobic respiration? 6. What are the reactants and products of cellular respiration? 1. 2. 3. 4. 7. About how many ATP molecules does a cell obtain from the breakdown of one molecule of glucose in cellular respiration? 8. Given the glucose, carbon dioxide and water, which one(s) is/are called high-energy molecule and which one(s) is/are called low-energy molecule? Suggested Answers: 1. Light-dependent reaction and light-independent reaction (also known as Calvin cycle reaction or carbon fixation reaction). 2. The basic stages of Calvin cycle reaction are: carbon dioxide fixation, carbon dioxide reduction, RuBP regeneration. 3. Reactants: carbon dioxide and water; products: carbohydrates and oxygen 4. Glycolysis happens in the cytoplasm of the cell; citric acid cycle (Krebs cycle) and ETC are in the mitochondrion of the eukaryotic cell. 5. In cellular aerobic respiration: three; in anaerobic respiration: one 6. Reactants: carbohydrates, oxygen, and about 38 ADP molecules; products: carbon dioxide, water and about 38 ATP molecules 7. About 36 to 38 ATP molecules (NOTE: This number is just a ratio. Some biology authors say there are 30, 32 or 34 ATP molecules produced depending on the shuttle used to transport the electrons and on the kind of species.) 8. High-energy molecules: glucose; low-energy molecules: carbon dioxide and water Directions: Fill-in the two tables below for the major events and features of photosynthesis and cellular respiration, respectively. The option tables are given for you to answer the needed materials and end products of photosynthesis and cellular respiration. 90 Major Events and Features of Photosynthesis Reaction Series Needed Materials End Products 1. Light-dependent reactions (take place in the thylakoid membrane) a. Photochemical reactions b. Electron transport c. Chemiosmosis a. a. b. b. c. c. 2. Carbon fixation reactions (take place in stroma) 2 2 Available Choices a. Electrons b. NADPH, O2 e. Electrons, NADP +, H O, electron 2 acceptors f. Proton gradient, ADP + P, ATP synthase c. Light energy; pigments (chlorophyll) g. Carbohydrates, ADP + P, NADP+ d. ATP h. Ribulose bisphosphate, CO2, ATP, NADPH, necessary enzymes Major Events and Features of Cellular Respiration Stage Starting Materials End Products 1. Glycolysis ( in cytosol) 2. Preparatory reaction 3. Citric acid cycle 4. Electron transport and chemiosmosis Available Choices a. Pyruvate, ATP, NADH e. Acetyl CoA, H2O, NAD+, FAD, ADP Pi b. NADH, FADH2, O2, ADP Pi f. Acetyl CoA, CO2, NADH c. Glucose, ATP, NAD +, ADP P i g. CO2, NADH, FADH2, ATP d. Pyruvate, Coenzyme A, NAD+ h. ATP, H2O, NAD+, FAD Suggested Answers: Major Events and Features of Photosynthesis Reaction Series Needed Materials End Products Light-dependent reactions (take place in the thylakoid membrane) a. Light-energy; pigments (chlorophyll) a. Electrons b. NADPH, O2 a. Photochemical reactions b. Electron transport Carbon fixation reactions (take place in stroma) b. Electrons, NADP+, H2O, electron acceptors c. Proton gradient, ADP + P, ATP synthase 2. Ribulose bisphosphate, CO2, ATP, NADPH, necessary enzymes c. ATP 2. Carbohydrates, ADP + P, NADP+ Major Events and Features of Cellular Respiration Stage Starting Materials End Products 1. Glycolysis (in cytosol) Glucose, ATP, NAD+, ADP Pi Pyruvate, ATP, NADH 2. Preparatory reaction Pyruvate, Coenzyme A, NAD+ Acetyl CoA, CO2, NADH 3. Citric acid cycle Acetyl CoA, H2O, NAD+, FAD, ADP Pi NADH, FADH2, O2, ADP Pi CO2, NADH, FADH2, ATP 4. Electron transport and chemiosmosis ATP, H2O, NAD+, FAD 92 Activity: Gaseous Products of Photosynthesis NOTE: If there is enough time and the materials are available, let the class do this activity. Materials needed: 1000 mL beaker, 3 grams of sodium bicarbonate, Hydrilla or Elodea, funnel, test tube Procedure: 1. Half-fill a 1000 mL beaker with tap water. 2. Add 3 grams of sodium bicarbonate. 3. Place Hydrilla or Elodea in the bottom of the beaker. 4. Put a funnel over the plant. 5. Fill the test tube with water up to the brim. Secure the mouth of the test tube with your thumb. Invert the tube and place it on top of the funnel. 6. Place the beaker under direct sunlight. Count the bubbles that appear in the test tube after 30, 60, 90, 120, 150, 180, and 210 seconds. 7. After several minutes, slowly remove the test tube from the funnel. Place your thumb over its mouth. Turn the tube right up and insert a glowing match to the test the presence of the oxygen in the tube. Adapted from: Science and Technology II for the Modern World. (2003). Makati City: Diwa Scholastic Press, Inc. Note: An illustration of the set-up will help teachers and students to visualize how the experiment should be performed. ENRICHMENT (25 MINS) Directions: Show the basic similarity and differences between photosynthesis and cellular respiration. The options are provided for in the other table below. PART I Photosynthesis Cellular Respiration 1. Raw materials 2. End products 3. Electron transfer compound 4. Location of electron transport chain 5. Organelle involved 6. ATP production 7. Source of electron for ETC 8. Type of metabolic reaction 9. Terminal electron acceptor for electron transport chain Available Choices a) c) e) O2 Glucose, oxygen NADP+ is turned to NADPH g) Phosphorylation and oxidative phosphorylation i) Chloroplast k) Photophosphorylation m) In noncyclic electron transport :H2O o) Glucose, oxygen q) In noncyclic electron transport: NADP+ b) d) f) Anabolism Carbon dioxide, water NAD+ is turned to NADH+ h) Mitochondrial inner membrane (cristae) j) Mitochondrion l) Thylakoid membrane n) Immediate source: NADH and FADH2 p) Catabolism r) Carbon dioxide, water 94 Suggested Answers Photosynthesis Cellular Respiration 1.Raw materials Carbon dioxide, water Glucose, oxygen 2.End products Glucose, oxygen Carbon dioxide, water 3.Electron transfer compound 4.Location of electron transport chain 5.Organelle involved NADP+ is turned to NADPH NAD+ is turned to NADH+ Thylakoid membrane Mitochondrial inner membrane (cristae) Mitochondrion 6.ATP production Photophosphorylation 7.Source of electron for ETC In noncyclic electron transport :H2O (undergoes photolysis to yield electrons, protons, and oxygen) Anabolism 8.Type of metabolic reaction 9.Terminal electron acceptor for electron transport chain Chloroplast In noncyclic electron transport: NADP+ (becomes reduced to form NADPH) Phosphorylation and oxidative phosphorylation Immediate source: NADH and FADH2, Ultimate source: glucose Catabolism O2 (becomes reduced to form H2O) Connecting the Concepts with the Biological Systems • • • Chloroplasts and mitochondria play a significant role in metabolism and their enzyme-requiring pathways permit a flow of energy through all living things. The energy transformations that take place in these organelles results in a loss of energy in the form of heat. Therefore, all organisms are in need of a constant supply of energy, which they get from their food. Food is ultimately produced by plants, which have the ability to capture solar energy. Photosynthesizing organisms form the basis of most food chains on Earth. EVALUATION (60 MINS) Suggested Answers: Directions: Summarize the similarity of the two organelles as they carry out opposite processes. PART II SET A: Revisit of Energy Organelles Structure 1. Use of Membrane 2. Electron Transport Chain 3. Enzyme Chloroplast Mitochondrion Electron Transport Chain SET B: Photosynthesis versus cellular respiration Directions: Using the following descriptions for photosynthesis and cellular respiration, bring out your long bond paper or Oslo paper, pencil, ruler, ballpen and coloring materials. Show an illustration/ diagram comparing the structure and function of chloroplasts and mitochondria. Label the parts of the organelles. The rubric for the drawing is given below. In Photosynthesis: • • • • Water is oxidized and oxygen is released Has electron transport chain located within the grana of chloroplasts, where ATP is produced by chemiosmosis Has enzyme-catalyzed reactions within the semi-fluid interior Carbon dioxide is reduced to a carbohydrate In Cellular respiration: • • • • Use of Membrane: In chloroplast • An inner membrane forms the thylakoid of the grana. In mitochondrion • An inner membrane forms the cristae. Oxygen is reduced to water Has electron transport chain located within the cristae of the mitochondria, where ATP is produced by chemiosmosis Has enzyme-catalyzed reactions within the semi-fluid interior A carbohydrate is oxidized to carbon dioxide 96 In chloroplast • An ETC is located on the thylakoid membrane. Electrons passed down the ETC have been energized by the sun; The ETC establishes an electrochemical gradient of H + with subsequent ATP production by chemiosmosis. In mitochondrion • An ETC is located on the cristae of mitochondrion. Energized electrons have been removed from glucose and glucose products. The ETC establishes an electrochemical gradient of H+ with subsequent ATP production by chemiosmosis. Enzymes In chloroplast • The stroma contains the enzymes of the Calvin cycle. In the Calvin cycle, NADPH and ATP are used to reduce carbon dioxide to carbohydrate. In mitochondrion • The matrix contains the enzymes of the citric acid cycle. In citric acid cycle, the oxidation of glucose products occurs as NADH and ATP are produced. Rubrics for the Drawing Standard Contrast and intensity of drawing Blending of colors Neatness Excellent (7 points) Good (5 points) Fair (3 points) Shows exceptional artistic and skillful color contrast; and meaningful color concentration Color mix is exceptionally creative, appropriate and meaningful Completely free from mess Shows generally acceptable artistic and skillful color contrasts; and meaningful color concentration Color mix is generally creative, appropriate and meaningful Almost free from mess Shows generally vague color contrasts; and indiscernible sense of color concentration Color mix needs improvement Too messy PART III Directions: Group the class into triad. Make each group construct a concept map to help them develop their understanding of photosynthesis and cellular respiration. Prepare a rubric for easy scoring. Standard Content knowledge Originality in organization of ideas Neatness Excellent (10 points) Good (7 points) Fair (4 points) Information is complete and accurate Exceptionally well organized and understandable Completely free from mess Information is mostly complete and accurate Generally well-organized and understandable Almost free from mess Information is mostly incomplete and inaccurate Fairly understandable Too messy PART IV Directions: Arrange the following to get the right energy flow sequence in aerobic respiration. NADH Electron Transport Chain Glucose Suggested Answers: 1. Glucose 2. NADH 3. Electron Transport Chain 4. ATP Directions: Identify the following statements as photosynthesis or cellular respiration. _______________1. Energy-releasing pathways _______________2. Energy-acquiring pathways Suggested Answers: 1. Cellular respiration 2. Photosynthesis 98 ATP General Biology 1 Forms of Energy, Laws of Energy Transformation and Role of ATP 120 MINS LESSON OUTLINE Introduction Communicate the learning objectives Motivation The “Nanay” Analogy 5 10 Content Standard The learners demonstrate an understanding on the forms of energy, laws on energy transformation, ATP Structure and Function and the ATP- ADP Cycle. Instruction/ a. Review on the idea of organisms as OPEN SYSTEMS Delivery 60 Learning Competency The learners: Practice 10 • Explain coupled reaction processes and describe the role of ATP in energy coupling and transfer STEM-bio11/12-IIa-j-2 Specific Learning Outcomes At the end of the lesson, the learners shall be able to: • • • • Identify different forms of energy in their surroundings. Present and explain a real life analogy of the ATP=ADP cycle. Relate the experiment done and the game to free energy and equilibrium; and ATP cycle respectively. Explain the topics discussed in their small groups (peer learning). b. Lesson Proper a. Hugot “Lines” b. ATP in everyday life Enrichment Small-group discussion (SGD) 15 Evaluation Quiz 20 Reflection End of topic question Materials Laboratory equipment needed, raw materials, school supplies Resources • Reece JB, Urry LA, Cain ML. 2010. Campbell Biology 10th. San Francisco(CA):Pearson Benjamin Cummings; 2010. pp. 141-151. INTRODUCTION (5 MINS) Teacher Tip: A. Communicate Learning Objectives (NOTE: PLEASE DO THE MOTIVATION BEFORE DOING THE INTRODUCTION) 1. Introduce the learning objective by writing it on the board, then give the students 5 minutes (to work in pairs) to write down on a piece of paper what they already know or what they expect to learn under the specified topics: • • • Through this introduction, you will have an idea where to start or how you will approach your discussion. You may also ask your students which topic would interest them the most and what would interest them the least. Forms of energy Laws of energy transformation Free energy and metabolism MOTIVATION (10 MINS) Teacher Tip: Start the motivation by reviewing/introducing the terms Metabolism, Anabolism, Catabolism, Bioenergetics, free energy, Entropy, Equilibrium, Digestion, Cellular Respiration, Photosynthesis and ATP. After refreshing the students with the terms and processes, group your students into groups of 3’s and allow them to think of how they can relate their mom (“NANAY”) to these processes. Have at least 3 groups of students share their analogy in front of the class. “Nanay” analogy example: Metabolism manages materials and energy resources of the cell. We can relate it to our mom/ Nanay because our mom is responsible most of the time in budgeting the finances and cooks food for the family.Entropy is the measure of disorder or randomness, Nanay makes sure that all the things inside the house are well organized especially after the kids played. Nanay lessens the house’s entropy after the increase in entropy done by the kids after playing by placing all the things in their proper places. Another set of analogies may include putting together the ingredients of a dish to create a palatable viand as an example of anabolism. Or removing one by one the parts of an engine to fix a broken car as an example of catabolism. 100 The teacher can also encourage the students to think of other analogies that will help them relate the lesson to their everyday lives. Please make sure that you look for the meaning of the terms mentioned and read about the topic to facilitate and make the discussion more interesting As long as the analogy relates to the concepts being discussed, give credits to your students. INSTRUCTION/DELIVERY (60 MINS) Start the discussion by establishing that living organisms are open systems (energy and matter can be transferred between the system and its surroundings). Remind the students that this was already mentioned in one of the characteristics of living organisms. Obtain and use materials for Energy, and in the unifying theme- interaction with the environment. This way students will clearly understand that processes (Bioenergetics) that happen within a living organism is still influenced and affects the surroundings. The teacher may directly involve students by pointing out that humans breath the carbon dioxide needed by plants as raw materials to produce food via photosynthesis, the teacher may use the figure below. Emphasize as well the role of oxygen as final electron acceptor in cellular respiration. Students should appreciate why they need to breathe in oxygen and not other form of gases. Teacher tip In the start of the lesson, since the terms metabolism, catabolism, anabolism were already used in the motivation, the teacher can explain it further by the use of the downhill and uphill metabolic avenues. Downhill avenue (Catabolism)- releases energy that will be used, enabling energy be stored. While Uphill avenue (Anabolism) uses energy to build complex materials. Solicit other examples from your students. Engage your students by giving examples first. If computers/laptops or lcd projectors are not available, the teacher may adjust the discussion by preparing materials written on a manila paper. The teacher may pave the discussion in such a way that the students will be answering or completing tables or having the students research (homework) about the topic. This will ensure that the students have something in line with the topic that will be discussed. Instead of pictures, the teacher can ask the students to do actual processes that will depict the different forms of energy Teacher tip Energy Flow and Chemical Recycling in Ecosystems Energy flows into ecosystem as sunlight and ultimately leaves as heat, while the chemical elements essential to life are recycled. Make sure that for every form of energy, you will be able to show a solid example in living systems how energy forms exists and transformed. Forms of Energy Teacher Tip Energy is the capacity to cause change. It is also the ability to rearrange a collection of matter. In the environment different forms of energy exist: Kinetic, Light and Potential energy. This part of the topic will really be the least of interest of the students. That’s why exploring the trend in terms of the “hugotlines” may help you in engaging students. Inform your students that at the end of the discussion you will be having the “hugot line” activity that will be based on the laws of thermodynamics. So they can start preparing for their “hugots” as you discuss the laws of thermodynamics. • • • • • Kinetic- energy associated with relative motion of objects Thermal energy-type of kinetic energy associated with random movement of atoms. When thermal energy is transferred in the form of heat. Light Energy- main energy source is the sun and powers photosynthesis (anabolic process). Potential Energy- possessed energy of a matter at rest (non- moving form) Chemical energy- potential energy released in a chemical reaction Laws of Energy Transformation Thermodynamics is the study of energy transformations that occurs in a system (collection of matter). Living systems are considered as open systems because energy and matter are transferred between systems and the surroundings. 1st Law: The energy of the universe is constant: Energy can be transferred and transformed but it cannot be created nor destroyed. Plants do not produce energy, but transforms energy from the sun. Some energy becomes unavailable to do work because most is lost as heat. Transfer of energy and transformation makes the matter more disordered. Disorder of matter is measured through entropy. The teacher may also incorporate the concept on pyramid of energy, where energy availability per trophic level decreases as one goes from produces to the top consumer. Only the energy stored by herbivores as biomass will be available to 1st order carnivores. 102 Laws of Energy Transformation Teacher Tip Thermodynamics is the study of energy transformations that occurs in a system (collection of matter). Living systems are considered as open systems because energy and matter are transferred between systems and the surroundings. Use the figure used, but make sure to note that an individuals’ contribution to the disorder in surrounding is not obviously felt, but as a group it is. Use the idea of having lots of people inside the room compared to an individual inside the room. 1st Law: The energy of the universe is constant: Energy can be transferred and transformed but it cannot be created nor destroyed. Plants do not produce energy, but transforms energy from the sun. Some energy becomes unavailable to do work because most is lost as heat. Transfer of energy and transformation makes the matter more disordered. Disorder of matter is measured through entropy. 2nd Law: Every energy transfer or transformation increases the energy of the universe. • • i.e In a room full of people, breathing increases entropy since all are exhaling carbon dioxide. Organisms as open system increase order as long as the order in their surroundings decreases. This shows that as living organism transfers/transforms energy to its surroundings, the disorder increases, thus increases entropy. Free Energy • • • • • • • • Energy that can do work under cellular conditions Gibbs free energy is the energy in the system that can perform work when temperature and pressure are uniform throughout the system: ∆G = ∆H – T∆S Also known as free energy change Measure of system’s instability (trend: tendency to change to a more stable state) Increase in G: UNSTABLE i.e. concentrated dye Decrease in G: STABLE i.e. dye dispersed in water In chemical reactions: as reaction precedes equilibrium, the free energy of reactants and products decreases (decreases free energy). If products will be removed free energy will increase When systems reach maximum stability, the system reaches the state of equilibrium. If equilibrium is reached there is NO WORK. In chemical reactions proceeding equilibrium NO NET CHANGE in the relative concentration of reactants and products. The teacher must take note that there is unstoppable trend towards randomization of the universe as a whole. ∆G = ∆H – T∆S, where ∆H is enthalpy (total energy), T is absolute temperature and ∆S is the change in entropy. If it is possible to show the concentrated dye experiment (diffusion of dye in water), it will be a big help. But before asking the students to do it, make sure that you prepare questions that will be answered to lead the students in the discussion of free energy. NOTE: You may have the first two bullets answered by the students before doing your discussion. Then after the discussion have the last 3 bullets answered. • • • • • Describe the free energy (G) of the concentrated dye? Does it have high or low free energy? Is it stable or unstable? When do we say that the dye reached the stable state? Is there a way for the diffused dye system to undergo spontaneous change? How will you do it? How will you relate the closed hydroelectric system to the set up of the dye diffusion experiment? • How will you relate the multistep hydroelectric system to the dye diffusion experiment? Free Energy and Metabolism Teacher Tip: • • Exergonic reactions- energy is released (energy outward), more decrease in free energy= more work done Endergonic reactions- energy is absorbed (energy inward). Plants stores energy in the form of glucose (from carbon dioxide and water Equilibrium and Metabolism • • • Equilibrium = NO WORK. This usually happened in isolated systems that reach equilibrium. A cell that reaches the state of equilibrium is DEAD A normal cell is not in equilibrium, because its products are not accumulated within its system, INSTEAD the products becomes a reactant in the next step. 104 It will be really helpful if you use the example of an isolated/closed hydroelectric system and an open hydroelectric system. A closed hydroelectric dam will reach equilibrium while the open hydroelectric system will not since the overspill will be used by another system. You may also think of other examples that are parallel to the hydroelectric system example. You may also use the dye diffusion experiment that you did in explaining the isolated hydroelectric system as example. Add a drop of concentrated dye to a glass of water. Since it is a closed hydroelectric system, the procedure stops there, where the dye already reached the state of equilibrium Adenosine Triphosphate (ATP) • • • • • • • • Structure composed of: sugar ribose, nitrogen base adenine and a chain of 3-phosphate groups Mediates most energy coupling in cells Powers cellular work 3 main kinds of work of a cell: chemical work, transport work and mechanical work. These are possible through energy coupling, where the cells use and exergonic process to drive an endergonic reactions. chemical work: synthesis of polymers from monomers (pushing of endergonic reactions) transport work: pumping of substances across membranes (against the direction of spontaneous movement) mechanical work: beating of cilia, contraction of muscles also used to make RNA (since ATP is used as one of the nucleoside triphosphate) ACTIVITY: Calamansi Relay ala ATP cycle Materials: Calamansi, plastic spoons (30) In an oval (if there is any in your area) or a lot where students can play the relay divide the class into 3 groups (10 members per group) assuming that there are 30 students in a class. Define who’s going to be number 1-10 (refer to the figure below). At your mark they will start the relay. Student 1 must be able to successfully pass the calamansi (calamansi on a plastic spoon bitten by student 1) to the students 2’s plastic spoon (must be bitten). The student’s hand must be at their back, NO USING OF HANDS. If the calamansi falls from the spoon the student has to go back to his/her starting point before proceeding in passing the calamansi to the next player. The group that will finish first will be the winner. The winner will be awarded with bonus points. Do the game before discussing the ATP hydrolysis and ATP cycle. Make sure that you read the game mechanics and do the adjustments to fit your classes. At the end of the activity discuss how it is related to ATP hydrolysis and ATP cycle. • Calamansi: water (for hydrolysis) • Students: phosphates that is cleaved Teacher Tip: The multistep hydroelectric system can be explained through the dye diffusion experiment by using more than one glass or water. You may show this by having the concentrated dye dropped to the first glass. Describe how the dye decreases free energy and how it becomes more stable by diffusing. But this doesn’t end with this set up, get another dropper and obtain a sample of the colored water from glass 1 then drop it into the clear water in glass 2. This way you are showing that the dye that had decreased its free energy and improved to be more stable can still undergo spontaneous change. Then repeat the procedure from glass 1 to glass 2 for the succeeding glasses. With this, you were able to show that a multi-step open hydroelectric system will not reach equilibrium because the product becomes the reactant for the next reaction. Teacher tip: The phosphate bonds of the ATP must not be termed as high-energy phosphate bonds. The bonds of the phosphate groups of the ATP are not strong bonds but instead, the reactants (ATP and water) possess high energy compared to the product. • Students exerting effort to reach the other end: energy releasing process to allow ADP + P to produce ATP (phosphorylation) • Bonus/incentive for the winner: energy • 2 groups: ATP and ADP Teacher Tip As for supplementary resource, you may look for videos that clearly explains the hydrolysis of ATP and the ATP Cycle. You may ask your students to view it before the class (HW) or have it shown in class as you discuss it. Hydrolysis of ATP • • • • • • process of breaking down bonds between the phosphate groups this happens when a water molecule breaks the terminal phosphate bond HOPO32-, abbreviated P I leaves ATP Forming Adenosine diphosphate (ADP) Energy is released. This comes from the chemical change of the system state of lower free energy and NOT from the phosphate bonds. Hydrolysis relases so much energy because of the lnegative charges of the phosphate groups. These charges are crowded together and their mutual repulsion contributes to the instability of that region of the ATP. The energy equivalent of the triphosphate tail of ATP is compared to a compressed spring. 106 Teacher Tip Look for videos showing the 3 cellular work powered by ATP. For the students to appreciate these processes more and for them to clearly see that its really happening in living systems. You may also use the lessons they had in endomembrane system and transport mechanisms to facilitate the discussion in this part of the topic. How the Hydrolysis of ATP Perform Work • • Proof that ATP releases heat: in a test set up, the hydrolysis of ATP releases energy in the form of heat in the surrounding water. Most of the time when an animal is exposed in a cold environment, the reaction of the body is through shivering. In this reaction of the organism, shivering uses ATP during muscle contraction to warm the body. Since it will also be a disadvantage for organisms to generate heat during ATP hydrolysis, in order to maintin the living conditions inside the cell, the energy released during ATP hydrolysis is used by proteins to perform work: chemical, transport and mechanical • Hydrolysis of ATP leads to change in the shape of protein and in its ability to bind to another molecule. Phosphorylation (ADP to ATP) and dephosphorylation (ATP to ADP) promote crucial protein shape changes during important cellular process The Regeneration of ATP • • • • • • • ATP is a renewable it can be regenerated by the addition of phosphate to ADP Catabolism (exergonic) provides the free energy to phosphorylate ADP. ATP formation is not spontaneous, so there is a need to use free energy for the process to work. ATP cycle is the shuttling of inorganic phosphate and energy. It couples the cell’s energy yielding processes (exergonic) to energy consuming process (endergonic) ATP regeneration happens very fast (10M molecules of ATP used ad regenerated per second) If ATP could not be regenerated by phosphorylation of ADP, HUMANS would use nearly their body weight in ATP each day. 108 PRACTICE (10 MINS) A. “Hugot-lines”. Create “hugot-lines” based on the laws of transformation of energy. Ask your students to create 3 “hugot-lines” per law under the transformation of energy. The student must give justification for each “hugot-line” they’ll create. Make sure that the “hugot-lines” are in tune with the scientific concepts of the law of thermodynamics. B. ATP in everyday life. Ask your students to make an analogy relating the concepts under ATP (ATP cycle, ATP hydrolysis, How ATP allows organisms to do work) or the relevance of ATP in our lives. After listing the analogies or the relevance of ATP in our lives, ask the students to write their reflection/ realization regarding the topic. ENRICHMENT (15 MINS) Small Group Discussion. Allow your students to meet in groups (5 students per group) Assign each students to a topic below: • Forms of energy • Transformation of energy • Free energy and metabolism • ATP- structure and function This is to see how much the students understood the lesson. Encourage your students to explain the concepts in their own words. You may allow you students to use their notes in this small discussion. If they have an access to internet and can bring their gadgets, you may also encourage them to save videos that will help them explain their topic. EVALUATION (20 MINS) The teacher can make his/her own list of questions that will allow students to practice critical thinking skills. Having this short quiz done by group of 2s or 3s will allow students to discuss and decide among themselves. If a student opts to answer individually, allow him/her. The teacher can prepare a multiplechoice type of evaluation if he/she is not comfortable with this type of exam 1. Explain metabolism through creating an appropriate analogy. 2. What is the difference between Catabolic and Anabolic reaction? How do each process compliment one another? 3. What is the importance of having exergonic reactions in the body? 4. What is the disadvantage if there are not enough endergonic reactions in the body? Relate this to the ATP cycle. 5. What happens when energy transforms to another form? Is the yield unchanging for every transformation? Why or Why not? 6. Which law of thermodynamics states that energy transfer or transformation increases the state of disorder of the universe? 7. Why do we consider living organisms as open systems? 8. If free energy is higher, does it mean that the system is more stable (Y/N) N? What does this imply in the amount of work that can be done? 9. If free energy is lower,does it mean that there is greater work capacity? (Y/N) N What does this imply about the systems stability? 10. How can you tell that a cell has reached equilibrium? What properties does it display? 11. (Y/N) Is the hydrolysis of ATP reversible? If Yes, explain your answer. 12. Describe how phosphorylation works (what drives it)? 13. How does the cell go about the continuous release of heat during ATP hydrolysis? 14. What will happen to the body if the regeneration of ATP is very slow? 15. How does the multi-step open hydroelectric system explain cellular respiration? Teacher Tip REFLECTION (HOMEWORK FOR THE NEXT MEETING) • • • • Which of the topics interest you the most? Why? Which of the topics interest you the least? Why? Did the activities help you understand the topic (Y/N)? Explain your answer. Did you see the significance/ connection of the topic in your life? 110 Encourage your students to answer this section truthfully as it will also help you improve the lesson and your approach. General Biology 1 240 MINS Energy Transformation Pt.1 Content Standard The learners demonstrate an understanding of photosynthesis. LESSON OUTLINE Introduction Assess prior knowledge through a class 10 Learning Competency The learners explain the importance of chlorophyll and other pigments (STEM_BIO11/12 – IIa-j-3) Motivation Laboratory activity on separating plant pigments through paper chromatography 50 Specific Learning Outcomes At the end of the lesson, the learners shall be able to: Instruction/ Discussion on chlorophyll, Delivery photoexcitation of chlorophyll, and the 120 • • • • separate and identify the different pigments present in plants through a simple paper chromatography experiment discuss the role of pigments in photosynthesis illustrate how chlorophyll absorbs and transforms light energy define and describe a photosystem activity on word clustering photosystem Practice Formative assessment through summary reporting 20 Enrichment Answering of critical thinking questions 20 Evaluation 20 Short quiz on topics discussed in the lesson Materials • laboratory materials and supplies for the chromatography experiment (i.e., chromatography or filter paper, glassware, acetone/solvent, spinach leaf, coleus leaf, coin, pencil, aluminum foil, ruler, and coffee stirrer) • prism Resources (1) Reece JB, U. L. (2010). Campbell Biology 10th. pp. 190 – 194. San Francisco(CA): Pearson Benjamin Cummings. (2) Starr, C. (2003). Biology: Concepts and Applications 5th edition. Pp.92 – 98. Belmont, California: Brooks/Cole-Tomson Learning. INTRODUCTION (10 MINS) Teacher Tip: 1. Assess the learners’ prior knowledge of the topic by facilitating a class activity on clustering or word webbing. Write the word CHLOROPHYLL on the board. This will serve as the ‘nucleus word’. 2. Ask the learners to think of words or images associated with the ‘nucleus word’ and let them write the words or sketch the images around the ‘nucleus word’. 3. Encircle each word or image and draw a line from each item to the ‘nucleus word’. 4. Ask the learners to write how each word or image is related to the ‘nucleus word’ CHLOROPHYLL and have them write the relation above or below the line that you drew. 5. Ask the learners to summarize the word web formed by the class. 6. After the summary, present the following questions to the class: • Why are chlorophyll and other plant pigments important? • How do chlorophyll and other plant pigments help in carrying out photosynthesis? By assessing the learners’ prior knowledge, you will be able to gauge what they already know and what they still need to learn from the lesson. This will help you design and determine the topics that you need to include and those that you need to reiterate and emphasize in your discussions. Posing essential questions at the start of a lesson helps stimulate thoughts, promotes inquiry, and motivates the learners to find and formulate ways to be able to come up with the correct responses. These essential questions shall help the learners focus in finding the correct responses. Teacher Tip: MOTIVATION (50 MINS) To help the learners acquaint themselves with different plant pigments, instruct them to perform a laboratory activity on chromatography of plant pigments. This activity will allow them to visually demonstrate that leaves contain different colored pigments. Before performing the experiment, provide a brief description of chromatography. Chromatography—is a separation technique used to identify various components of mixtures based on the differences in their structure and/or composition. It involves a stationary phase (e.g., paper or any thin layer of an absorbent surface) and a mobile phase (i.e., solvent containing the dissolved substances). The solvent will move up the paper through capillary action carrying with it the dissolved substances. These substances will be carried along at different rates because they are not equally soluble in the solvent and they will be attracted in different degrees to the paper. Provide the learners with a copy of the instructions for the activity. Guide them as they perform the experiment. 112 Use the following guidelines in performing the laboratory activity: • Make sure that the learners have knowledge of safety practices in the laboratory. • Let the learners work in groups in order to smoothly carry out the experiment. • Prepare the materials a day before the activity. Extracting Plant Pigments through Chromatography Objective: Perform chromatography to separate a mixture of pigments from plants Materials per group: • Chromatography paper strips or filter paper about 1cm x 15cm in size • Acetone (solvent) • 150 ml beaker or a tall glass jar • aluminum foil to serve as cover for the beaker • fresh spinach leaf • Coleus leaf or other leaf that is red in color • coin • pencil • ruler • coffee stirrer or plastic stick Procedure (Adapted with modifications from <http://www.dal.ca/content/dam/dalhousie/pdf/faculty/science/imhotep/9.2-Discovering-PlantPigments.pdf>): 1. Using a pencil, draw a base line that is 2cm from the bottom of the paper strip. Be careful in handling the chromatography paper as oil from the human skin can alter the results. Lift the paper only by its sides and be careful not to touch its front. 2. Place the spinach leaf over the paper. Pressing hard, roll the edge of the coin, and rub the leaf onto the paper, following the path of the line. Repeat until the line turns very dark. 3. Repeat the same process for the Coleus leaf using a second strip of chromatography paper. 4. Add enough acetone to cover the bottom of the beaker or glass jar (no more than 1cm high). 5. Attach the top of the paper strips to a pencil or a coffee stir stick. This can be done by making a loop with the top of the paper and fastening it with a paper clip or tape. 6. Lay the pencil or stick across the top of the beaker so that it suspends the paper above the liquid. The bottom of the paper strip must be dipped in the solvent but the solvent should not surpass the 2cm baseline that is the point of origin. 7. Cover the beaker and allow 15–30 minutes for the solvent to rise through the strips. 8. Remove the paper strips just before the solvent reaches the top. 9. Lay the paper strip face up. Using the pencil, immediately mark the line where the solvent stopped before it evaporates. This is called the solvent front. 10. Allow the strips to dry. 11. Before the pigments fade, mark the top of each color that you can identify. 12. Measure the distance (in mm) travelled by each pigment from the point of origin. 13. Tabulate your data. Show the following information in your table: color observed, distance travelled, and probable pigment. INSTRUCTION/DELIVERY/PRACTICE (120 MINS) Teacher Tip: Using the learners’ observations from the experiment and the data they have gathered, have them discuss their analysis and conclusion among their groups with the help of these guide questions: • • • • • The learners’ discussion of the experiment’s result, analysis, and conclusion will jumpstart the lecture and discussion on pigments and their role in photosynthesis. Which pigments were you able to observe in your chromatogram? Why do the pigments move at different rates through the chromatogram? How do the spinach leaf and Coleus leaf differ from each other in terms of their pigments? Which of the two leaves can carry out photosynthesis better? Why? Why is it an advantage for plants to have different colored pigments? Let the groups share their analysis and conclusion in class. Discuss the following concepts: Teacher Tip: Pigments Pigments are substances that absorb visible light. Different pigments absorb light of different wavelengths. 114 If a prism is available, you may use it to demonstrate the various colors of visible light. Light, as it encounters an object, is either reflected, transmitted, or absorbed. Visible light, with a wavelength of 380–750nm, is the segment in the entire range of electromagnetic spectrum that is most important to life on earth. It is detected as various colors by the human eye. The color that is not absorbed by pigments of objects is transmitted or reflected and that is the color of the object that we see. !!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!Figure'1:!The!Electromagnetic!Spectrum! Pigments are the means by which plants capture sun’s energy to be used in photosynthesis. However, since each pigment absorbs only a narrow range of wavelength, there is usually a need to produce several kinds of pigments of different colors to capture more of sun’s energy. Chlorophyll Chlorophyll is the greenish pigment found in the thylakoid membrane inside the chloroplast of a plant cell. The figure below shows the location and structure of a chloroplast. Teacher Tip: Encourage participation from the students while discussing these concepts. Chlorophyll absorbs blue and red light while it transmits and reflects green light. This is why leaves appear green. There are several kinds of chlorophyll. Among these, chlorophyll a plays the most important role in photosynthesis. It directly participates in converting solar energy to chemical energy. Other pigments in the chloroplast play the part of accessory pigments. These pigments can absorb light and transfer the energy to chlorophyll a. One of these accessory pigments is chlorophyll b. Some carotenoids also contribute energy to chlorophyll a. Other carotenoids, however, serve as protection for chlorophyll by dissipating excessive energy that will otherwise be destructive to chlorophyll. Structure of chlorophyll • Head—a flat hydrophilic head called porphyrin ring. It has a magnesium atom at its center. Different chlorophylls differ on the side groups attached to the porphyrin. • Tail—a lipid-soluble hydrocarbon tail. How does photoexcitation of chlorophyll happen? 1. A chlorophyll molecule absorbs photon or light energy. 2. An electron of the molecule in its normal orbital, said to be in its ground state, will be elevated to an orbital of a higher energy. The molecule is now in an excited state. The molecule only absorbs photon that has the energy that is equal to the energy needed for it to be able to elevate from the ground state to the excited state. 3. The excited state is unstable. Hence, excited electrons drop back down to the ground state immediately after, releasing energy in the form of heat and photon. This happens in isolated chlorophyll molecules. However, chlorophyll molecule that is found in its natural environment in the thylakoid membrane forms a photosystem together with proteins and other organic molecules to prevent the loss of energy from the electrons. 116 Teacher tip For this part, you may ask some learners to volunteer to show the location of chlorophyll in a leaf of a plant. They may do this through drawings or sketches in the board. Teacher tip Reiterate the importance of the formation of photosystem. The formation of the photosystem prevents the excited electrons from going back to the ground state, thus preventing the loss of energy which is essential for photosynthesis to occur. Figure 4: Photoexcitation of Chlorophyll Photosystem A photosystem is an aggregate of pigments and proteins in the thylakoid membrane responsible for the absorption of photons and the transfer of energy and electrons. It is composed of: • Light-harvesting complex— is also called the ‘antenna’ complex and is consisted of several different pigments (chlorophyll a, chlorophyll b, and carotenoids) bounded with proteins. When a pigment molecule absorbs a photon, energy is passed on from one pigment molecule to another pigment molecule until the energy reaches the reaction center. • Reaction-center complex—is composed of a pair of chlorophyll a and a primary electron acceptor. The primary electron acceptor is a specialized molecule that is able to accept electrons from the pair of chlorophyll a. The pair of chlorophyll a in the reaction-center is also specialized because they are capable of transferring an electron to the primary electron acceptor and not just boosting the electron to a higher energy level. There are two types of photosystem: • • Photosystem II—was discovered later after the discovery of Photosystem I, but functions first in the light reaction of photosynthesis. The chlorophyll a in the reaction-center of Photosystem II effectively absorbs light with a wavelength of 680nm and thus called P680. Photosystem I—was discovered first. Its reaction-center has a chlorophyll a called P700 because it is effective in absorbing light with a wavelength of 700nm. PRACTICE (20 MINS) Teacher tip 1. Divide the class into three groups. Assign each topic you discussed to each group: chlorophyll and other pigments, photoexcitation of chlorophyll, and photosystem. 2. Instruct each group to perform a Summary Report of the topic assigned to them. Each member of the group will have to say something about the topic without consulting their notes. 3. Give the groups enough time to organize and consolidate their report before asking them to present in class. ENRICHMENT (20 MINS) Ask the learners to answer the following questions: • • In places where there are four seasons (i.e., winter, spring, summer, and fall), how do plants cope with the change in season? Give a detailed description and explanation. Chlorophyll-enriched products and supplements are now being sold in the market. Find out what benefits these products claim. With your knowledge about chlorophyll, do you think these claims are valid? Support your answer. Ask the learners to share their answers in class. 118 This activity will serve as a formative assessment to evaluate the learners’ understanding of the lesson. This will also provide you an opportunity to reinforce concepts that are not clearly understood and to rectify misconceptions, if there are any. EVALUATION (20 MINS) Give the students a short quiz to assess their understanding of the topics discussed in the lesson. You may formulate other questions that can gauge their learning. What happens to light when it hits an object. What wavelength of light is most important to life on earth? How do plants capture the sun’s energy? In what cell organelle can we find chlorophyll? In what structure of this organelle is chlorophyll located? 5. What color/s of light does chlorophyll absorb? What color does it reflect? 6. What might be the advantage of accessory pigments? 7. What happens to a chlorophyll molecule when it absorbs photons? 8. How do chlorophyll molecules prevent the loss of energy when electrons go back to the ground state? 9. What composes a photosystem? 10. In what part of the photosystem does the first step of light reaction takes place? 11. Differentiate the two types of photosystem. 1. 2. 3. 4. General Biology 1 180 MINS Energy Transformation Pt.2 Content Standard The learners demonstrate an understanding of photosynthesis. LESSON OUTLINE Learning Competency The learners describe the patterns of electron flow through light reaction events (STEM_BIO11/12–IIa-j-4) Introduction Communicating learning competencies 20 Motivation 10 and outcomes; Familiarization with key terms Specific Learning Outcomes At the end of the lesson, the learners shall be able to: • • • differentiate the two stages of photosynthesis identify the important molecules involved in the light reactions describe the events and processes happening during light reactions Establishing the importance of photosynthesis to all organisms by using plant samples Instruction/ Discussion on the events of light Delivery reactions. 60 Practice 60 Role-playing activity Enrichment Think-pair-share class activity 10 Evaluation 20 Quiz on the light reactions events diagram Materials • samples and photos of plant products (e.g. fruits and vegetables) • materials for the role-playing activity (e.g. tennis balls or soft balls, lids, and labels) Resources (1) Reece JB, U. L. (2010). Campbell Biology 10th. pp. 190 – 194. San Francisco(CA): Pearson Benjamin Cummings. (2) Starr, C. (2003 ). Biology: Concepts and Applications 5th edition. Pp.98 - 100. Belmont, California: Brooks/Cole-Tomson Learning. 120 INTRODUCTION (20 MINS) 1. Communicate the learning competencies and learning outcomes of this lesson to the class. 2. Give the learners enough time to research on the definition and description of the following keywords: • light reactions • noncyclic electron flow • cyclic electron flow • plastoquinone (Pq) • plastocyanin (Pc) • ATP • photophosphorylation • ferredoxin • NADP+ • NADPH • chemiosmosis MOTIVATION (10 MINS) 1. Establish to the learners that photosynthesis is important not only to plants but also to other living organisms such as humans. 2. Show pictures of food with vegetables or fruits to the class. You may also bring real fruits and vegetables to the class. Let the learners identify them. 3. Ask the learners what percentage of their diet consisted of fruits and vegetables. Ask learners to make conclusions on the role played by plants in converting the sun’s energy to a form of energy that the human body can use.Using a pencil, draw a base line that is 2cm from the bottom of the paper strip. Be careful in handling the chromatography paper as oil from the human skin can alter the results. Lift the paper only by its sides and be careful not to touch its front. Teacher Tip: Communicating the learning competencies and learning outcomes shall give the learners an idea on what they can expect to learn from the lesson. You may also opt to establish prior understanding of the topic or lesson. You can ask the learners to show specific hand signals depending on how familiar they are on the topics to be discussed. For example, they can do a ‘thumbs up’ to indicate extensive understanding of the specific topic, a ‘thumb to the side’ to signify enough understanding of the topic, and a ‘thumbs down’ to show little or no understanding at all. INSTRUCTION/DELIVERY (60 MINS) Teacher Tip: You may the students to write on the board the chemical reaction for photosynthesis. Review the chemical reaction for photosynthesis: 6 CO2 + 6 H20 + sunlight C6H12O6 + 6 O2 Ask the learners to identify which among the reactants is/are used in the light reactions and the Calvin cycle. Give an overview and further differentiate the two stages of photosynthesis. • • Light reactions—use sunlight to initiate electron transfer, thereby reducing NADP+ to NADPH and splitting water to give off oxygen as a by-product. • form ATP through phosphorylation • take place in the thylakoids of the chloroplast Calvin Cycle—sometimes referred to as ‘dark reactions’ because it does not require light energy for its processes to take place • incorporates CO2 into organic molecules through carbon fixation • uses NADPH and ATP to produce carbohydrate from the fixed carbon • takes place in the stroma of chloroplast • returns ADP, inorganic phosphate, and NADP+ to the light reactions Show an image of the light reactions similar to the one shown in Figure 2 below: 122 Using a diagram showing an overview of photosynthesis, have the students figure out differences between the two stages involved. Teacher Tip: While discussing the events in the light reaction, make sure to engage the learners and encourage them to participate by asking questions before proceeding with the discussion on each step or event. The following could be sample questions: • What happens to electrons when they encounter light energy? • How is oxygen formed in the first phase of light reaction? • What happens to the hydrogen ions formed from the splitting of water molecules? Teacher Tip: Figure 2: The Light Reactions From the image or diagram of the light reactions, ask the learners to identify the key players involved in the process. You may review the concept of redox reaction in this part. In an oxidation reaction, a molecule loses one or more electrons and becomes more positively charged. In a reduction reaction, a molecule gains one or more electrons and becomes more negatively charged. Oxidation and reduction reactions are most often paired, resulting to a redox reaction. As a molecule is oxidized, the molecule that accepts the electrons is reduced. Teacher Tip: Proceed to an in-depth discussion of the steps or events in light reactions. Light Reactions Events 1. Light energy or photon is absorbed by a pigment molecule of the light-harvesting complex of Photosystem II and is passed on to other pigment molecules nearby until the energy makes it to the reaction center. In the reaction center, it is absorbed by the P680 pair of chlorophyll a. Integrate the First Law of Thermodynamics which states that energy can be transferred or transformed from one form into another but cannot be created nor destroyed. Ask the students how the First Law of Thermodynamics is applied in Photosynthesis. Ask the learners to cite specific events in the process that illustrates the law. 2. The electron in this pair of chlorophyll a is raised to an excited state and is transferred to the primary electron acceptor. P680 loses its electron and becomes positively charged (P680+). 3. The positively charged molecule attracts electrons from a water molecule, resulting to the splitting up of H20 into two electrons, two hydrogen ions (H+), and an oxygen atom with the provision of light energy. The oxygen atom immediately combines with another oxygen atom to form an oxygen molecule (O2) which is then released outside the leaf through the stomata. 4. The excited electrons are then passed on from the primary electron acceptor to the electron carrier molecules through the electron transport chain until they reach Photosystem I. The electron carrier molecules involved here are plastoquinone (Pq), a cytochrome complex, and plastocyanin (Pc). 5. At each transfer, the electrons release small amounts of energy. This energy is used to pump hydrogen ions across the membrane. The splitting up of water molecules results to an uneven distribution of hydrogen ions in the stroma and the lumen. The H+ ions tries to equalize their distribution by moving from the lumen to the stroma through the aid of a membrane protein called ATP synthase. This is referred to as chemiosmosis. The movement of hydrogen ions through the ATP synthase channel triggers the synthesis of ATP from ADP. The ATP contains high-energy phosphate bonds. 6. Meanwhile, photon is also absorbed and energy is passed on from one pigment molecule to another until the energy reaches the reaction center complex of Photosystem I. The energy excites the electron present in the pair of P700 chlorophyll a located here. The excited electron is then transferred to a primary electron acceptor, making the P700 positively charged and now seeking electrons to fill up the missing ones. This is filled up by the electrons from Photosystem II that are passed on through the electron transport chain. 7. The photo-excited electron from the primary electron acceptor of Photosystem I enters another electron transfer chain, passing the electron to an iron-containing protein called ferredoxin (Fd). 8. An enzyme, the NADP+ reductase, then transfers the electron to NADP+ and stabilizes it by adding a proton (H+) to form NADPH. NADPH is then released to the stroma and becomes part of the Calvin Cycle. Cyclic Electron Flow Aside from the usual route of electron flow as described in the events of the light reactions (i.e., noncyclic or linear electron flow), photo-excited electrons may take a short-circuited route which utilizes Photosystem I but not Photosystem II. The ferrodoxin goes back to the cycle and passes the electron 124 to the cytochrome complex and to the Pc until it reaches P700 chlorophyll instead of transferring the electron to NADP+reductase. Due to this event, no NADPH is produced but ATP is still synthesized. Figure 3: Cyclic Electron Flow PRACTICE (20 MINS) Teacher tip LIGHTS, Camera, ReACTIONS! 1. Take the class outside the classroom and look for a wide area that will allow the learners to move around. 2. Ask the learners to pick a role as players in light reaction events (e.g., hydrogen ions, oxygen atoms, etc.). 3. Let the learners plan how they are going to act out the process. 4. You may provide the following information and materials to assist them in planning and performing the roleplaying activity. Materials: • balls (tennis balls or soft balls) that will serve as electrons • lids or any material that will serve as light energy or photon • labeled cards for the following roles: sun • pigment molecules (several learners) • water molecule (i.e., oxygen atom and hydrogen ions) • P680 • primary electron acceptors • electron carriers (i.e., Pq, cytochrome complex, Pc, and Fd) • ADP • P700 • NADP+ reductase • NADP+ Ask the class present their simulation of the light reactions. 6. After the presentation, provide feedback, if there is any. This activity shall help the learners process the information that they acquired. This shall ensure that the learners acquire a deeper understanding of the concepts. Through this activity, you shall have an idea of how well the learners understood the topics you discussed. It shall give you the chance to correct any misunderstandings and misconceptions they might have regarding the lesson. • 5. For this part of the lesson, the learners shall take on roles and act out the events that happen during light reactions. This shall be a class effort and shall need each learner’s cooperation to be able to accurately act out the events. Learners who are not directly involved in the performance shall serve as director and scriptwriters to ensure the smooth flow of the ‘play’. 126 ENRICHMENT (60 MINS) Think-Pair-Share 1. First, instruct the learners to determine what event/s in the light reactions they consider to be the most significant to humans. Teacher tip You may also opt to formulate questions to be answered as a quiz. The quiz may be done individually or in pairs. 2. Next, ask the learners to look for a partner and share their response. 3. Then, ask the pairs to share their responses to the whole class. Pair: Look for a partner and discuss your answers. EVALUATION (20 MINS) Assess the learners’ understanding of the lesson by having them accomplish the diagram below. They need to fill in the empty shapes with the names of the molecules involved in the processes. This activity may serve as a quiz. Assign a homework to the class. Ask the learners to conduct a research and answer this question: What do you think is the importance of the Cyclic Electron Flow to the process of photosynthesis and to the cell? General Biology 1 240 MINS Energy Transformation Pt.3 Content Standards The learners demonstrate an understanding of photosynthesis. LESSON OUTLINE Learning Competency The learners describe the significant events of the Calvin Cycle (STEM_BIO11/12-IIa-j5) Introduction Communicating learning competency 10 Motivation 20 and learning outcomes; Description of the stages of the Calvin Cycle Specific Learning Outcomes At the end of the lesson, the learners shall be able to: • • • describe the phases of the Calvin cycle identify the important molecules needed in the Calvin cycle identify the molecules produced in the cycle Familiarization with key words and researching for their meanings or definitions Instruction/ Discussion on the three phases of Calvin Delivery cycle 60 Practice 60 Building of a three-dimensional model of the Calvin Cycle Enrichment Researching and reporting on the 60 Evaluation 30 photosynthetic adaptations of C3, C4, and CAM plants Class quiz Materials • materials for making three-dimensional models of the Calvin Cycle (e.g., clay, Styrofoam balls, beads, and art materials) Resources (1) Reece JB, U. L. (2010). Campbell Biology 10th. San Francisco(CA): Pearson Benjamin Cummings. (2) Starr, C. (2003). Biology: Concepts and Applications 5th edition. Belmont, California: Brooks/Cole-Tomson Learning. 128 INTRODUCTION (10 MINS) Teacher tip 1. Present the learning competency and learning outcomes for this lesson. 2. Describe the significant events of the Calvin cycle. 3. Review the basic features of the Calvin cycle. Let the learners provide the overview or description of the Calvin cycle. You may refer to the equation of photosynthesis in introducing the topic. The Calvin Cycle • also referred to as light-independent reactions or “dark reactions” • takes place in the stroma of the chloroplast • second stage of photosynthesis that is involved in the formation of sugar from CO2 using chemical energy stored in ATP and NADPH, the products of light reactions MOTIVATION (20 MINS) 1. Write the following terms on the board: CO2 • RuBP • ATP • Rubisco • NADPH • 3-phosphoglycerate (PGA) • G3P • 1,3-biphosphoglycerate 2. Explain to the learners that these terms are the key players in the Calvin cycle that they need to familiarize themselves with. 3. Instruct the learners to research the meaning and description of the molecules involved in Calvin cycle. INSTRUCTION/DELIVERY (60 MINS) Give a lecture-discussion on Calvin cycle. The Calvin Cycle Important points to know: • The sugar that is produced in the Calvin Cycle is not the six-carbon glucose that we are familiar with. This is formed later on. What is produced in the Calvin Cycle is a three-carbon sugar known as G3P or glyceraldehyde-3-phosphate. • The Calvin Cycle needs to ‘spin’ three times to make one molecule of G3P from three molecules of CO2. Three Phases of Calvin Cycle: Carbon Fixation • • • Carbon fixation is a process of incorporating an inorganic carbon molecule, CO2, into an organic material. In this phase, the CO2 molecule is attached to a five-carbon sugar molecule named ribulose biphosphate (RuBP) aided by an enzyme named rubisco or RuBP carboxylase. Rubisco is believed to be the most abundant protein in the chloroplast and maybe on Earth. The resulting product, a six-carbon sugar, is extremely unstable and immediately splits in half. The split forms two molecules of a 3-phosphoglycerate (3-carbon). Reduction • • • • • A phosphate group (from ATP) is then attached to each 3-phosphoglycerate by an enzyme, forming 1,3-phosphoglycerate. NADPH swoops in and reduces 1,3-biphosphogycerate to G3P. For every six G3Ps produced by the Calvin Cycle, five are recycled to regenerate three molecules of RuBP. Only one G3P leaves the cycle to be packaged for use by the cell. It will take two molecules of G3P to make one molecule of glucose. The ADP and NADP+ that is formed during the Calvin Cycle will be transported back to the thylakoid membrane and will enter the light reactions. Here, they will be ‘recharged’ with energy and become ATP and NADPH. 130 Teacher Tip: You may also opt to have the learners make a graphic organizer that shows the three phases of the Calvin Cycle. Regeneration of RuBP • Five molecules of G3P undergo a series of complex enzymatic reactions to form three molecules of RuBP. This costs the cell another three molecules of AT, but also provides another set of RuBP to continue the cycle. What happens to G3P after its release from the cycle? • Two G3Ps can combine together to form either glucose or fructose which are both are six-carbon sugar. • Glucose and fructose can be combined to form sucrose. • Glucose can be connected in chains to form starch. • G3Ps can also be used in lipid and protein synthesis. The cost of making carbohydrate: To make one molecule of G3P, the chloroplast needs: • 3 molecules of CO2 • 9 molecules of ATP • 6 molecules of NADPH PRACTICE (60 MINS) Divide the learners into five or six groups. Orient the class on the nature of the activity, as described below: Calvin Cycle 3-D Model Building For this activity, each group has to gather materials that will help them build a three-dimensional model that represents the events or phases of the Calvin Cycle. You may use clay, Styrofoam balls, beads, or recyclable materials. The outputs will be presented in class. ENRICHMENT (60 MINS) • • • • Ask the learners to work in groups for this activity. Instruct them to research on photosynthetic adaptations of plants found in desert environments. Assign the following topics for their research outputs: C3 plants, C4 plants, and CAM plants. The groups may choose any method of presenting their outputs. They can opt for oral presentations, role-playing, poem or song making, or using visual arts and media. EVALUATION (30 MINS) Ask the learners to answer the following questions: • Temperature and light intensity are two of the factors that may affect the rate of photosynthesis. Explain how the said factors affect photosynthesis. Which of the two would you expect to have more effect on the rate at which the Calvin Cycle proceeds? Why? • Many urban areas in our country are becoming less eco-friendly in exchange for new buildings and commercialization. What do you think is the implication of this in relation to photosynthesis? • With your knowledge on photosynthesis, correlate the process in helping curb the effects of climate change. 132 General Biology 1 360 MINS Energy Transformation - Cellular Respiration (Part 1 of 3) Content Standard The learners demonstrate an understanding of cellular respiration. Learning Competencies The learners: • • Differentiate aerobic from anaerobic respiration (STEM_BIO11/12-IIa-j-6) Describe the role of oxygen in cellular respiration and describe pathways of electron flow in the absence of oxygen (STEM_BIO11/12-IIa-j-10) Specific Learning Outcomes At the end of this lesson, the students must be able to; 1. determine the functional definition of cellular respiration; 2. compare fermentation with anaerobic and aerobic respiration; 3. explain how cells can produce ATP in the presence or absence of oxygen; 4. identify the metabolic pathways where aerobic respiration specifically occurs; and 5. explain how the lack of oxygen in the body results in eventual death of an organism. LESSON OUTLINE Introduction As part of understanding by design, engage 5 the students in learning exploring and firming up. Motivation To extend and refine your students’ understanding on energy transformation, you may ask them regarding the function and structure of the mitochondrion Instruction/ A series of activities with directions are Delivery indicated below. Practice Post guide questions Enrichment Answer the modified true or false, make a graphic organizer on aerobic and aerobic respiration and accomplish the Venn diagram Evaluation Answer the multiple-choice questions and a diagram that shows a comparison among fermentation, aerobic and anaerobic respiration 10 15 240 60 30 Resources • Enger, Eldon D. et. al., (2012). Concepts in Biology 14th Edition. USA: McGraw-Hill • Mader, Sylvia S. (2010). Biology 10th Edition. USA: McGraw-Hill • Mader, Sylvia S. (2013). Biology 11th Edition. USA: McGraw-Hill • Reece, Jane B. et al., (2011). Campbell Biology 9th Edition. San Francisco USA: Pearson Education, Inc. • Solomon, Eldra P. et al., (2008). Biology 8th Edition. China: Thomson Brooks/Cole INTRODUCTION (5 MINS) As part of learning exploration, let your students define cellular respiration. You may also ask students to describe process happening when they respire using their respiratory system then connect this process of respiration to what is happening inside the cell (cellular respiration). Cellular respiration by technical definition includes both aerobic and anaerobic respiration processes. But today cellular respiration is often used to refer to aerobic processes. MOTIVATION (10 MINS) To help your students analyze and identify attributes and components of the mitochondrion, ask them the following questions: 1. What are the major parts of the mitochondrion? 2. What is the function of each part? 3. What would happen if each part were missing? 4. What is your conclusion? Give examples of answers from students and what conclusion you expect to give a good motivation to students INSTRUCTION/DELIVERY (15 MINS) Procedure 1. Determine and list the molecules that enter and the molecules that leave the metabolic pathways of aerobic cellular respiration. 2. The pictures below can be redrawn or printed so that the students can visualize the metabolic pathways of the glucose molecule. 3. Post the redrawn visual learning materials on the board. Tell the students to work this out individually. Metabolic Pathways Glycosis Reactants and Products Molecules that enter: Molecules that leave: Krebs cycle Molecules that enter: Molecules that leave: Electron Transport Chain Molecules that enter: Molecules that leave: Provide expected answers to the table 134 Teacher tip Teacher Tip: To fill-out the table above, you may opt to give hint to your students: Glycolysis: three molecules Krebs cycle: three molecules those that enter and four those that leave ETC: three molecules ergy forms exists and transformed. Courtesy: Enger, Eldon D. et. al., (2012). Concepts in Biology 14th Edition. USA: McGraw-Hill (Retrieved August 13, 2015.) PROCEDURE 1. To extend and refine your students’ knowledge on cellular respiration, tell them to do the sample graphic organizer below. 2. Fill-out the table and distinguish how the two types of respiration are alike and different. Then tell them to write their conclusion based on the similarities and differences they have listed. 3. You may form three groups for this activity. Each group has to present the output(s) to the class using any kind of visual learning materials. Comparing Graphic Organizer AEROBIC RESPIRATION ANAEROBIC RESPIRATION How alike? AEROBIC RESPIRATION ANAEROBIC RESPIRATION How different? Summary and Conclusion 136 AEROBIC RESPIRATION ANAEROBIC RESPIRATION How alike? Both undergo glycolysis in the cytoplasm of the cell Both undergo substrate-level phosphorylation and oxidative phosphorylation and chemiosmosis in producing ATP molecules Both split the 6-carbon glucose into two molecules of pyruvate, the three-carbon molecule Both involve a series of enzyme-controlled reactions that take place in the cytoplasm Both use NAD+ (nicotinamide adenine dinucleotide), a redox coenzyme that accepts two electrons plus a hydrogen (H+) that becomes NADH Both performed by eukaryotic and prokaryotic cells AEROBIC RESPIRATION ANAEROBIC RESPIRATION How different? Maximum yield of 36 to 38 ATP molecules per glucose Complete breakdown of glucose to carbon dioxide and water with the use of oxygen Multiple metabolic pathways Pyruvate proceeds to acetyl formation in the mitochondrion The presence of enough oxygen in the cell makes the cell perform its job smoothly without burning sensation More efficient in harvesting energy from glucose with estimated 39% energy efficiency (36-38 ATP) in eukaryotic organisms but much higher ATP production (38 to 40 ATP) in prokaryotic organisms Outputs are carbon dioxide, water and ATP Products produce are for biochemical cycling and for the cellular processes that require energy Slow glucose breakdown Electrons in NADH are transferred to electron transport chain Mechanism of ATP synthesis is by substrate-level and oxidative phosphorylation/chemiosmosis Maximum yield of 2 ATP molecules per glucose for obligate anaerobes Partial degradation of glucose without the use of oxygen (obligate anaerobes) Single metabolic pathway (in fermentation) Pyruvate is broken down to ethanol and carbon dioxide or lactate (in fermentation) Cause burning sensation in the muscle during strenuous exercise (in fermentation) Less efficient in harvesting energy from glucose with 2% energy efficiency (for obligate anaerobes) Outputs are lactate, alcohol and carbon dioxide (in fermentation); but reduced inorganic compound in anaerobic respiration Produce numerous products with economic and industrial importance through fermentation Rapid breakdown of glucose Electrons in NADH are transferred to electron transport chain; but in fermentation electrons in NADH are transferred to organic molecule Mechanism of ATP synthesis is by substrate-level and oxidative phosphorylation/chemiosmosis; but in fermentation substrate-level phosphorylation only during glycolysis O2 is the final electron acceptor of the electron transport system Brain cells in the human body can only live aerobically. They die if molecular oxygen is absent. In anaerobic respiration, inorganic substances like NO3- or SO42- are the final acceptor of the electron transport system; but in fermentation, there is no electron acceptor because it has no electron transport system. Some organisms like yeasts (eukaryotic), many bacteria (prokaryotic) and the human muscle cells (eukaryotic) can make enough ATP to survive in facultative anaerobes (can live in the absence or presence of oxygen). But under anaerobic conditions lactic acid fermentation occurs. A facultative anaerobe needs to consume the nutrient at a much faster rate when doing the fermentation or anaerobic process. Summary and/or Conclusion Aerobic respiration requires molecular oxygen to happen in the cells of most eukaryotes and prokaryotes. Here, nutrients are split into a series of enzyme-controlled reactions producing an estimated 36 to 38 ATP per glucose complete breakdown. Molecular oxygen is the final acceptor of the low-energy level electron at the end of the electron transport system that results in the production of water. In anaerobic respiration on the other hand does not require oxygen in splitting nutrients. Some prokaryotes that live in oxygen-free environments such as water logged soil, in ponds where water does not flow, and in the intestines of animals transfer glucose to NADH and then pass the electrons down the electron transport chain that is joined to ATP synthesis by chemiosmosis. Nitrate and sulfate are the final acceptors of electrons. The end products are carbon dioxide, reduced inorganic substances and ATP. In fermentation (as type of anaerobic respiration) there is no electron acceptor because it has no electron transport chain. Its products are either alcohol (and carbon dioxide) or lactate. Note: Clarify how many minutes should be allotted for this activity ETC: A Metaphor PROCEDURE 1. To describe how the electron transport system performs its function along the cristae (folds) of the mitochondrion, the students will prepare the following materials: coloring materials, Manila paper(s), color papers, markers, pencil, and ruler. 2. The learners will make an analogy or a metaphor on how the electrons are being passed on to electron transport chain that result in the release of water. 3. In their drawing, they have to illustrate the participation of NADH, FADH2, hydrogen proton ion, electrons and oxygen along the electron transport system. Review your students that the simultaneous cooperation of these carrier molecules and hydrogen atoms are being used to run ATP production by chemiosmosis. They have to show that ATP and water are two of the products of ETC. 138 4. To facilitate their understanding, you can give them metaphoric examples such as bucket relay for ETC and a stair. A sample illustration is given below for your reference. 5. Form four groups for this activity. You may form rubric for this part focusing on appropriateness of illustration with all key ideas and elements are put together correctly. Stair image courtesy of: Mader, Sylvia S. (2013). Biology 10th Edition. USA: McGraw-Hill (Retrieved August 15, 2015.) Directions: The pictures below describe the pathways of electron in the absence of oxygen. Analyze it by arranging the seven metabolic pathways from numbers 1 to 7 provided for you in the opposite table. The same procedure is followed in another table for fermentation with numbers 1 to 6. Images courtesy of: Mader, Sylvia S. (2013). Biology 11th Edition. USA: McGraw-Hill (Retrieved August 17, 2015.) 140 Metabolic Pathways Outside the Mitochondria: Glycolysis (Note: This is not sequenced.) Metabolic Pathways Outside the Mitochondria: Glycolysis (Note: Arrange the pathways in order from 1 to 7.) 1 Water is released as 3PG is oxidized. 1 + 2 G3P is oxidized as NAD receives high energy-electrons 2 coming from the hydrogen atoms of C6H12O6. 3 Substrate-level ATP synthesis occurs. 4 Two Pyruvate molecules (3-carbon) are produced as the end products of glycolysis. 5 Splitting of the 6-carbon sugar produces 3-carbon molecules. 6 Substrate-level ATP synthesis occurs (also called as substrate-level phosphorylation). 7 Two ATP molecules are used to start glycolysis. Metabolic Pathways Outside the mitochondria: Fermentation (Note: This is not sequenced.) G3P is oxidized as NAD+ receives high energy-electrons coming from the hydrogen atoms of C6H12O6. NAD+ is “freed” to return to the glycolytic pathway to pick up more electrons. Two ATP molecules are used to start glycolysis. Two molecules of pyruvate are converted to ethanol (with CO2 as by-product) and lactate. Splitting of BPG into two molecules of pyruvate is couple to substrate-level ATP synthesis. Splitting of the 6-carbon sugar produces 3-carbon molecules. 3 4 5 6 7 Metabolic Pathways Outside the Mitochondria: Fermentation (Note: Arrange the pathways in order from 1 to 6.) PRACTICE (240 MINS) 1. Explain how NAD+, pyruvate, oxygen and ATP are involved in aerobic cellular respiration. 2. What is the role of oxygen in cellular respiration. 3. What are the members of the chain in the electron transport system? 4. What do the cristae (or folds) in the mitochondrion contain? 5. What happens to the hydrogen ions (H+) carried by NADH and FADH2? 6. Contrast the energy-investment step with the energy-payoff step of glycolysis. 7. How is aerobic cellular respiration different between prokaryotic and eukaryotic organisms? 8. What happens during electron transport and what it has to do with a proton pump? 9. Using arrows show in simple diagram the metabolic for glycolysis. 10. Explain how ATP can continue to be produced in the absence of oxygen. Suggested Answers: 1. NAD+ accepts electrons and delivers them to the ETS. Pyruvate is the product of glycolysis. It is converted to acetyl-CoA and transferred to the Krebs cycle. Oxygen is the final electron acceptor of the ETS and combines with hydrogen to form water. ATP is used in glycolysis to get the process going but ultimately it is the most valuable molecule produced by aerobic respiration. All parts of aerobic respiration result in a net yield of ATP. 2. Oxygen molecule is the final acceptor of electrons from ETC. It receives the low energy electron from the last of the carriers (that is, cytochrome oxidase). After receiving electrons, the oxygen molecule combines with hydrogen ions, and water is formed. 3. The members of the chain in sequence are the following: NADH-Q reductase, coenzyme Q, cytochrome reductase, cytochrome c, cytochrome oxidase. These are the members of the chain which accept high-energy level electrons which they pass from one molecule to another. 4. The cristae contain the chain members (carrier molecules and protein complexes, ATP synthase complex and ATP channel protein (bulk of ATP is produced by chemiosmosis). 5. The complexes of the ETC use the released energy to pump these hydrogen ions from the matrix into the intermembrane space of mitochondria. 6. If you want to earn, you really need to invest, and therefore you need a capital of some amount. During the energy-investment step, 2 ATPs are used to split glucose into 2 pyruvate molecules. The split of glucose produces a gross of 4 ATPs and 2 NADH. 4 ATP- 2 ATP = 2 ATP net in the glycolysis. 7. Prokaryotic organisms do not have mitochondria. These organisms use a slightly different way to perform the Krebs cycle and ETC that results in slightly more ATP than is produced by eukaryotic organisms. 142 8. The electron transport chain consists of a series of molecules which accept electrons and transfer them from one molecule to another. As electron is passed on along the series, energy is released to run ATP production. As this happens, protons are pumped from one location to another in the mitochondrion. Protons begin to build up in their new location. This creates a chemical gradient producing a bulk of ATP by chemiosmosis. These ATP molecules can be used by the cell to do work. 9. Glucose G3P BPG 3PG PEP pyruvate 10. ATP can still be produced without oxygen. This can be done through anaerobic fermentation. A net of 2 ATP molecules are produced during glycolysis. Glucose proceeds through the glycolysis pathway, producing pyruvate. This process “frees” NAD+ and it returns to the glycolytic pathway to up more electrons to become NADH again. ENRICHMENT (60 MINS) Directions: This is a modified true or false activity. Write the word TRUE if the underlined word/phrase being referred to is correct. If it is false, change the word/phrase to make the whole statement correct based on the concept of cellular respiration. Write your answer on the space provided before each number. _________1. Fermentation and anaerobic respiration enable the cells to produce ATP without the use of oxygen. _________2. The term cellular respiration includes both aerobic and anaerobic processes. _________3. Fermentation is a complete degradation of sugars or other fuel that occurs without the use of oxygen. _________4. An electron transport system consists of a number of molecules, majority are proteins, located in the matrix of the mitochondria of eukaryotic cells and the plasma membrane of aerobic prokaryotes. _________5. Pyruvate oxidation and the citric acid cycle, oxidative phosphorylation: electron transport chain and chemiosmosis are the metabolic stages reserved for cellular respiration. _________6. The breakdown of glucose to carbon dioxide is completed in the electron transport chain. _________7. ATP synthase is the enzyme that makes the bulk of the ATP from ADP and Pi by chemiosmosis _________8. ATP synthase uses the energy of an existing hydrogen ion gradient to power ATP synthesis. _________9. Phosphorylation of ADP to form ATP stores at least 14.6 kcal per molecule of ATP. ________10. Citric acid cycle generates 2 ATP whether oxygen is present or not, whether the conditions are aerobic or anaerobic. Suggested Answers: 1) True 2) True 3) Partial or incomplete 4) Cristae or folds 5) True 6) Krebs cycle 7) True 8) True 9) 7.3 kcal 10) glycolysis Directions: Accomplish the table below by comparing aerobic and anaerobic respiration Factors Aerobic Respiration Anaerobic Respiration Factors Aerobic Respiration Anaerobic Respiration Main function Production of ATP without the use of oxygen Site of Reaction Production of ATP Sustainability Production of lactic acid Oxygen requirement Recycling of NADH Production of ATP from food such as carbohydrate, lipid and protein Cytoplasm and mitochondrion 36 to 38 ATP per glucose molecule Long-term Does not produce Yes Through the electron transport system Participating cells Most cells Main function Site of Reaction Production of ATP Sustainability Production of lactic acid Oxygen requirement Recycling of NADH Participating cells Suggested Answers: 144 Cytoplasm 2 ATP per glucose molecule Short-term Produces No In lactic acid fermentation (i.e., muscle cells; in alcohol fermentation (pyruvate is converted to carbon dioxide and ethanol) Yeast, other fungi, prokaryotes, muscle cells Directions: Compare aerobic and anaerobic respiration by accomplishing the Venn diagram below. Venn Diagram of Aerobic and Anaerobic Respiration EVALUATION (30 MINS) Directions: Compare fermentation with anaerobic and aerobic respiration by analyzing the diagram below. Diagram courtesy of: Enger, Eldon D. et. al., (2012). Concepts in Biology 14th Edition. USA: McGraw-Hill (Retrieved August 13, 2015) 1. What are the three kinds of enzyme-controlled reactions so that the chemical-bond energy from a certain nutrient is released to the cell in the form of ATP? 2. What are the hydrogen electron acceptors for aerobic and anaerobic respiration as well as in fermentation? 3. These are the by-products of aerobic respiration that are considered low-energy molecules. 4. What are the outputs produced by anaerobic respiration? What about in fermentation? 5. What are two general metabolic mechanisms by which certain cells can oxidize organic fuel and generate ATP without the use of oxygen? 146 Suggested Answers: 1. Aerobic respiration, anaerobic respiration, and fermentation 2. aerobic respiration — molecular oxygen, anaerobic respiration — nitrate or sulfate, fermentation – pyruvate 3. Water and carbon dioxide 4. Anaerobic respiration—ATP, water reduced acceptor (nitrate or sulfate), fermentation, ATP, carbon dioxide, alcohol or lactate 5. Anaerobic respiration and fermentation Directions: This is a multiple-choice task. Encircle the letter of the correct answer. 1. Majority of the CO2 is released during a. Glycolysis b. Citric acid cycle c. Electron transport chain d. Oxidative phosphorylation 2. Cellular respiration processes that do not use O2 are called a. heterotrophic organism. b. anaerobic organism. c. aerobic organism. d. anabolic 3. The positively charged hydrogen ions that are released from the glucose during cellular respiration eventually combine with _________ ion to form ______ a. another hydrogen, a gas b. a carbon, carbon dioxide c. an oxygen, water d. a pyruvic acid, lactic acid 4. The Krebs cycle (also known as citric acid cycle or tricarboxylic acid) and ETC are biochemical pathways performed in which eukaryotic organelle? a. nucleus b. ribosome c. chloroplast d. mitochondrion 5. Anaerobic pathways that oxidize glucose to generate ATP energy by using an organic molecule as the ultimate hydrogen acceptor are called a. fermentation. b. reduction. c. Krebs cycle. d. Electron pumps 6. When skeletal muscle cells function anaerobically, they accumulate the compound________, which causes muscle soreness. a. pyruvic acid b. malic acid c. carbon dioxide d. lactic acid 7. Each molecule of fat can release ______ of ATP, compared with a molecule of glucose. a. smaller amounts b. the same amount c. larger amount d. only twice the amount. 148 8. In complete accounting of all ATPs produced in aerobic respiration, there a total of ________ATPs: ______ from the ETC, _____ from glycolysis, and _______ from the Krebs cycle. a. 36, 32, 2, 2 b. 38, 34, 2, 2 c. 36, 30, 2, 4 d. 38, 30, 4, 4 9. The chemical activities that remove electrons from glucose result in the glucose being a. reduced. b. oxidized. c. phosphorylated. d. hydrolyzed. 10. Which of the following is NOT true of the citric acid cycle? The citric acid cycle a. includes the preparatory reaction b. produces ATP by substrate-level ATP synthesis c. occurs in the mitochondria d. is a metabolic pathway, as is glycolysis Suggested Answers: 1. b 2.b 3.c 4.d 5.a 6.d 7.c 8.a 9.b 10. a General Biology 1 420 MINS Energy Transformation Cellular Respiration (Part 2 of 3) Content Standard The learners demonstrate an understanding of cellular respiration. • Introduction Communicate to the class the learning Explain the features and sequence the chemical events of cellular respiration (STEM_Bio11/12-IIa-j-7) Distinguish major features of glycolysis, Krebs cycle, electron transport system, and chemiosmosis (STEM_Bio11/12-IIa-j-8) Specific Learning Outcomes At the end of this lesson, the students must be able to: 1. Identify the major stages of cellular respiration; 2. Identify the organelles involved for each stage of cellular respiration. 3. Describe the following for each stage of cellular respiration: process, starting materials, and end products of aerobic respiration. Motivation Show a picture of students eating at the school canteen, then show questions 15 250 Enrichment Do the jigsaw activity and people 90 Evaluation 60 hunting and applying knowledge of biochemical pathways Fill-in the necessary information for cellular respiration and the 3-2-1 Closing Resources • • • Mader, Sylvia S. (2010). Biology 10th Edition. USA: McGraw-Hill Reece, Jane B. et al., (2011). Campbell Biology 9th Edition. San Francisco USA: Pearson Education, Inc. Solomon, Eldra P. et al., (2008). Biology 8th Edition. China: Thomson Brooks/Cole Suggested Media Tools: • www.mhhe.com/maderbiology11 • www.masteringbiology.com • www.biologycorner.com For animations, video, learning outcomes, chapter outline, image PowerPoint, essay quiz, thinking critically, practice test and concept maps http://highered.mheducation.com/sites/0073403466/student_view0/ chapter7/image_powerpoint.html http://highered.mheducation.com/sites/0073525502/student_view0/ chapter7/index.html 150 5 competencies. Review the reactants and products of cellular respiration Instruction/ A 3-D model of a mitochondrion made Delivery from re-usable materials Learning Competencies The learners: • LESSON OUTLINE INTRODUCTION (5 MINS) Teacher tip You may ask them the following questions: Emphasize that both prokaryotic and eukaryotic organisms need food in order to get the energy needed to adapt to many things in the environment and to perform bodily processes such as growth, repair, reproduction, maintenance of homeostasis, etc. Communicate to the class the learning competencies. Then go over the reactants and products of cellular respiration. 1. How many molecules of ADP as reactant are needed to produce about 38 molecules of ATP for eukaryotic organisms? 2. Which groups in the cellular respiration equation go in? 3. Which groups are released? Suggested Answers: 1. About 36 to 38 ADP molecules (NOTE: This number is just a ratio. Some biology authors say there are 30, 32 or 34 ADP (or ATP) molecules depending on the shuttle used to transport the electrons and on the kind of species.) 2. Groups that go in: carbohydrate and molecular oxygen 3. Groups that are released: carbon dioxide, water and energy (ATP) NOTE: Cellular respiration is one of the more difficult topics in biology. To capture the general picture of the topic, students have to be encouraged to read and re-read the key concept, write and re-write, outline and re-outline, draw and re-draw, and to recite orally if they want the ideas to sink in their system. Patience and steadfastness are important virtues that should be included as you study this concept. MOTIVATION (15 MINS) Post a color picture of a group of students eating at the school canteen. To establish healthy academic atmosphere and camaraderie, ask them if they know one who is a friend of them. Then ask the following questions: 1. If one of the students who ate would pay to the cashier a bill in US dollar, would the cashier accept the money as a form of payment for the food ordered? Suggested Answers: 1. No 2. Encash the 1000-peso cheque first at the bank. 3. Convert the US dollar bill to Philippine Peso and encash the 1000-peso bill cheque at the bank. 4. ATP or Adenosine Triphosphate, a form of nucleic acid 2. If one of the students ate combo meal and the amount of the food eaten is P49.00 and he gave out 1000-peso money cheque to the cashier, what do you think the cashier would ask to the student? (Assuming that the student is the first customer of the day). 3. What should the students do (one with a US dollar bill and one with a 1000-peso money cheque) to make their money more functional? 4. Just like the US dollar bill and the 1000-peso money cheque, the glucose (carbohydrate) in the food that we eat is a principal high-energy molecule that has to be digested into smaller molecules in order to release the high energy molecule that is highly recognized by the cell. What do you call this molecule that serves as the “energy currency of the cell”? 5. After this group of students ate the food at their school canteen, how do they obtain energy from these food (protein, carbohydrate, fat) molecules? Process the answers of the students related to the US dollar bill. Give the relationship of this analogy to the current topic. INSTRUCTION/DELIVERY (250 MINS) Activity 1: 3-D Mitochondrion Model 1. Let your class form triad. Give a model color picture of a mitochondrion that will serve as their guide for the 3-D mitochondrion. Print color pictures of a mitochondrion and give one to each group. 2. To make a 3-D model of a mitochondrion, prepare the following materials: Old newspapers, metal wires, starch (serves as paste), vinegar, acrylic paint, and paintbrush. 3. The sizes of the 3-D model mitochondrion are: Length — 24 inches, width — 14 inches, height — 8 inches. 4. From the metal wire, mold a mitochondrion based on the sizes given. The cristae of the mitochondrion can be formed through the metal wire. 5. Prepare a mixture for the starch (this will serve as your paste) as follows: 2 cups water, 2 cups starch, 1 cup vinegar. Mix the materials together under normal fire for 3-5 minutes. 6. With the old newspapers collected from the students’ neighbors, tell your students to wet the newspapers with paste and glue them on to the metal wire. 7. Let the model dry up before they paint the 3-D model mitochondrionProvide expected answers to the table 152 5. The complex food molecules are broken down by digestion into simpler substances that are absorbed by the body through the bloodstream. These food molecules will then be transported to all their cells. Breaking down of food is a catabolic process that converts the energy in the chemical bonds of nutrients to chemical energy stored in ATP that occur inside the cells of these students. This process in known as cellular respiration. NOTE: The US dollar currency is not directly used in everyday transaction. It has to be converted into usable form such as the Philippine money. Cellular respiration is different from organismic respiration. The latter refers to the exchange of gases such as oxygen and carbon dioxide with the environment by living organisms, particularly animals with special organs such as lungs and gills, for gas exchange.) Teacher Tip Inform the groups of students one week prior to this topic to give way to the preparation and designing of a 3-D model of a mitochondrion. You may form three groups. Give each group a model color picture of a mitochondrion that will serve as their template for the 3-D. Activity 2: Drawing, Coloring and Labeling 1. Inform your students to bring the following materials: Oslo paper (or long bond paper), coloring materials, pencil and a ballpen. 2. Let them draw and color, and label the model picture of a mitochondrion below. 3. The rubric for the drawing and coloring is given to help in the objective scoring of the output coming from your students. 4. The students will proceed to labeling. There are 10 blanks to be filled-in. 5. Then proceed to discussion by giving them processing questions. Rubrics for Drawing and Coloring Standard Contrast and intensity of drawing Blending of colors Neatness Excellent (7 points) Good (5 points) Fair (3 points) Shows exceptional artistic and skillful color contrast; and meaningful color concentration Color mix is exceptionally creative, appropriate and meaningful Completely free from mess Shows generally acceptable artistic and skillful color contrasts; and meaningful color concentration Color mix is generally creative, appropriate and meaningful Almost free from mess Shows generally vague color contrasts; and indiscernible sense of color concentration Color mix needs improvement Too messy Teacher tip ̌For the drawing, coloring and labeling of the parts of a mitochondrion, you may print the picture in color and post it on the board for everyone to see. Model Picture of a Mitochondrion Courtesy: Solomon, Eldra P. et al., (2008). Biology 8th Edition. China: Thomson Brooks/Cole (Retrieved July 20, 2015) Activity 3: Sequencing Cellular Respiration Teacher tip Procedure: 1. Group the class into three. Fill-in the missing information to make the graphic organizer complete. Few examples are given. 2. Report the output to the class. You will be graded according to the following rubric. NOTE: Students might find this difficult to accomplish. To facilitate better understanding for this 154 Insight: The reason why we humans breathe is to get the oxygen into our cells. Recall that at the end of the electron transport chain, hydrogen ions are being welcomed by the oxygen molecules to produce a by-product called water. activity, tell them to research on the major events of cellular respiration (e.g. how glucose gets into the cell and how it becomes converted into ATP). Your students should be able to see the major events (though the events are happening simultaneously). You may also prepare a set of reading materials (including diagrams and/or pictures) given to each group and let every member of the group analyze the text and be able to sequence and narrate the major events of cellular respiration. Suggested Rubrics Standard Excellent (10 points) Good (7 points) Fair (4 points) Content knowledge Information is complete and accurate Information is mostly complete and accurate Originality in organization of ideas Exceptionally well organized and understandable Generally well-organized and understandable Information is mostly incomplete and inaccurate Fairly understandable STAGE 1: Glycolysis This is what happens: During this stage, glucose (the six-carbon molecule) is split into two molecules of pyruvate (which contains three carbons). A net of two ATP molecules is produced by substrate-level phosphorylation during glycolysis. In addition, there are four hydrogen atoms are removed and are used to produce two NADH (an electron-carrier molecule that enters mitochondrion Note: You can say more at this stage. Courtesy: Solomon, Eldra P. et al., (2008). Biology 8th Edition. China: Thomson Brooks/Cole (Retrieved August 2, 2015) STAGE 2: ____________________ This is what happens: STAGE 3: ____________________ This is what happens: 156 STAGE 4: ______________________ This is what happens: Activity 4: Watch Summary Video for Aerobic Respiration (If materials are available) Cellular Respiration Video\www.youtube.comwatchv=00jbG_cfGuQ.mp4 (Retrieved August 3, 2015) Cellular Respiration Video\www.youtube.comwatchv=-Gb2EzF_XqA.mp4 (Retrieved August 3, 2015) Directions: With the help of your instructional materials (e.g. in tarpaulin form, PowerPoint Presentation or even simple pictures that are visually attractive and accurate) ask the following processing questions. Allow the pictures and the boardwalk to speak to your students. Processing Questions: 1. How many metabolic pathways are present in aerobic respiration? 2. Where in the cell part does glycolysis take place? What about the formation of Acetyl CoA, Krebs cycle and the electron transport chain and chemiosmosis? 3. How many reduced NADH molecules are produced after the glucose has been completely broken down to ATP? And what stage of the aerobic respiration is glucose completely broken down to carbon dioxide? 4. As glucose is split in the cytosol of the cell, is there a release of carbon dioxide as by-product of the reaction? 5. What molecule accepts the hydrogen atoms at the end of electron transport chain? 6. What is the major goal of NADH and FADH2 in aerobic respiration? 7. Why do you think the cell needs to digest glucose or any other nutrients such as protein and fats? 8. Among the metabolic pathways of cellular respiration, which phase is the major contributor of ATP? 9. What happens to pyruvate if oxygen is not available in the cell? 10. How many acetyl-CoAs are produced from each glucose molecule? Suggested Answers: 1. Three metabolic pathways 2. Glycolysis takes place in the cytoplasm or cytosol; formation of acetyl CoA, Krebs cycle, ETC and chemiosmosis all take place in the mitochondrion 3. 10 NADH molecules; glucose is completely broken down carbon dioxide at the Krebs cycle 4. No 5. Oxygen molecule 6. The goal of NADH and FADH2 is to transport the electrons coming from the hydrogen atoms (in glucose) to the electron transport chain 7. The cell has to digest glucose, fat, and protein in order to convert them into usable form of energy molecule called adenosine triphosphate. This ATP is the only molecule that is recognized by the cell for all of its cellular activities. 8. Among the metabolic pathways in aerobic respiration, the electron transport chain (or oxidative phosphorylation) makes about 90 per cent of ATP per glucose molecule. 9. The pyruvate will not proceed to the formation of acetyl CoA. The pyruvate will become lactic acid in animal and alcohol in plant. 10. Two 158 ENRICHMENT (90 MINS) Directions: Read the procedures below on how the jigsaw and expert groups are formed. PART I: Jigsaw Activity Procedure: 1. Form a group having four members. Each student-member in the group will be assigned to a certain phase of cellular respiration (1. glycolysis, 2. preparatory reaction, 3. citric acid cycle, 4.electron transport chain). Each group will be called “jigsaw group”. 2. Each group will be given handouts (with texts and pictures/diagrams—samples of these pictures are shown below) with the help of its leader and distribute them to his/her group mates. The handouts contain information about cellular respiration. Other helpful tools such as biology textbook, Internet can be used to facilitate their learning of the topic. 3. All the members in each group will be given enough time to read over their assigned topic for them to become familiar with it. 4. From the “jigsaw group” previously formed, the so-called “expert group” will then be formed. Those assigned in glycolysis from each group will be grouped as “expert group” in glycolysis. The same procedure will be done to preparatory reaction, citric acid cycle and electron transport chain — “expert groups” will be formed from these remaining topics. 5. These “expert groups” will be given enough time to discuss the main points of their assigned topic and to rehearse for the presentation. 6. After a certain amount of time, each student from the “expert group” will go back to his/her “jigsaw group”. 7. Each member from the “jigsaw group” will this time begin to present his/her topic to the whole class. Teacher tip • • • • Encourage each member to ask question(s) for clarifications. Provide cellular respiration handouts/ diagrams for each group to discuss. For this part, provide a rubric for the presentation that each group has to make. Writing the definitions of the terms can help your students better understand the concept. • Glycolysis – means “sugarsplitting” that occurs in the cytosol of the cell. It does not require oxygen to breakdown glucose into pyruvate. • Krebs cycle – completes the metabolic breakdown of glucose to carbon dioxide and produces 2 ATP. • Oxidative phosphorylation – a process occurring in mitochondria and accounts for majority of the ATP production. • Electron Transport Chain – contains the chain members (carrier and protein complexes, ATP synthase complex and ATP channel protein. These membrane proteins shuttle electrons during the redox reactions. The electrons will be used to produce ATP by chemiosmosis. • • NADH and FADH2 – these are electron acceptor molecules that contain high-energy electrons. They transport the electrons to ETC to produce many more ATPs by oxidative phosphorylations. ATP synthase – is an enzyme that is responsible for the great production of ATPs. This happens when it uses the energy coming from H+ ions to bind ADP and phosphate group together to produce ATP. Teacher Tip The diagram on the left shows the total energy produced from the complete breakdown of glucose by aerobic respiration. Rubric for the Report Standard Excellent (10 points) Good (7 points) Fair (4 points) Content knowledge Information is complete and accurate Originality in organization of ideas Exceptionally well organized and understandable Information is mostly complete and accurate Generally wellorganized and understandable Information is mostly incomplete and inaccurate Fairly understandable 160 PART II People Hunt 1. Prepare four sets of paper strips. Set A contains the four stages of cellular respiration. Set B contains the summary for each stage. Set C contains starting materials for each stage. Set D contains end products for each stage. 2. Twelve students will be asked to volunteer. Randomly, each of these volunteers will be given strips of paper (NOTE: tell them not to read yet the information written on the strip of paper). 3.Student-volunteers will be asked to scatter around the room. After this, instruct them that there are four stages of cellular respiration (please refer back to the procedure number 1). The student-volunteers will be given time to find their group mates correctly for each specific stage based on the paper strip(s) they are holding. 4. Tell each group for each stage to line up at the four corners of the room (north, south, east, west side of the room) and check if each member has found their group mates correctly. Summary of Cellular Respiration Stage Summary Some Starting Materials Some End Products 1. Glycolysis (in cytosol) Series of reactions in which glucose is degraded to pyruvate; net profit of 2 ATPs; hydrogen atoms are transferred to carriers; can proceed anaerobically Glucose, ATP, NAD+, Pi Pyruvate, ATP, NADH 2. Formation of acetyl CoA Pyruvate is degraded and combined with coenzyme A to form (in mitochondria) acetyl CoA; hydrogen atoms are transferred to carriers; CO2 is released Pyruvate, coenzyme A, NAD+ Acetyl CoA, CO2, NADH 3. Citric acid cycle (in mitochondria) Series of reactions in which the acetyl portion of acetyl CoA is degraded to CO2; hydrogen atoms are transferred to carriers; ATP is synthesized Acetyl CoA, H2O, NAD+, FAD, ADP, Pi CO2, NADH, FADH2, ATP 4. Electron transport and chemiosmosis (in mitochondria) Chain of several electron transport molecules; electrons are passed NADH, FADH2, O2, ADP, Pi along chain; released energy is used to form a proton gradient; ATP is synthesized as protons diffuse down the gradient; oxygen is final electron acceptor ATP, H2O, NAD+, FAD Applying Knowledge of Biochemical Pathways As scientists have developed a better understanding of the processes of aerobic cellular respiration and anaerobic cellular respiration, several practical applications of this knowledge have developed: • Although for centuries people have fermented beverages such as beer and wine, they have often plagued by sour products that were undrinkable. Once people understood that there were yeasts that produce alcohol under anaerobic conditions and bacteria that converted alcohol to acetic acid under aerobic conditions, it was a simple task to prevent acetic acid production by preventing oxygen from getting to the fermenting mixture. • When it was discovered that the bacterium that causes gas gangrene uses anaerobic respiration and is, in fact, poisoned by the presence of oxygen, various oxygen therapies were developed to help cure patients with gangrene. Some persons with gangrene are placed in hyperbaric chambers, with high oxygen levels under high pressure. In other patients, only the affected part of the body is enclosed. Under such conditions, the gangrene-causing bacteria die or are inhibited. • Spoilage, or putrefaction, is the anaerobic respiration of proteins with the release of nitrogen and sulfur-containing organic compounds as products. Protein fermentation by the bacterium Clostridium produces foul-smelling chemicals such as putrescine, cadavarine, hydrogen sulfide, and methyl mercaptan. Clostridium perfringens and C. sporogenes are the two anaerobic bacteria associated with the disease gas gangrene. A gangrenous wound is a foul-smelling infection resulting from the fermentation activities of those two bacteria. • Because many disease-causing organisms are prokaryotic and have somewhat different pathways and enzymes than do eukaryotic organisms, it is possible to develop molecules, antibiotics that selectively interfere with the enzymes of prokaryotes without affecting eukaryotes, such as us humans. • When physicians recognized that the breakdown of fats releases ketone bodies, they were able to diagnose diseases such as diabetes and anorexia more easily, because people with these illnesses have bad breath. • In starvation and severe diabetes mellitus, the body does not metabolize sugars properly, and it shifts to using fats as its main source of energy. When this occurs, the Krebs cycle is unable to perform as efficiently and the acetyl CoA does not move into the mitochondria. It accumulates in the blood. To handle this problem, the liver converts acetyl CoA to ketone bodies (e.g., acetoacetic acid). As ketone bodies accumulate in the blood, the pH decreases and the person experiences ketosis, or ketoacidosis, with symptoms such as an increased breathing rate; in untreated cases, it can lead to depression of the central nervous system, coma, and death. Adapted from: Enger, Eldon D. et al., Concepts in Biology 14th edition. USA: McGraw-Hill 162 EVALUATION (60 MINS) Teacher tip Directions: Complete the tables below by filling-in the necessary information for aerobic respiration. Table 1: Inputs and Outputs of Glycosis Glycosis Inputs Table 1 Suggested Answers: Glycolysis outputs: • 2 pyruvate • 2 NADH • 2 ADP • 4 ATP total • 2 ATP net gain Outputs Table 2 Suggested Answers: Citric Acid Cycle inputs: • 2 acetyl groups • 6 NAD+ • 2 FAD • 2 ADP + 2 P 1. Glucose 1 2. 2 NAD+ 2 3. 2 ATP 3 4. 4 ADP + 4 P 4 Table 3 Suggested Answers: Total: First Column Glycolysis-ATP-SLP= 2 ATP net Krebs-ATP-SLP= 2 ATP Total-ATP-SLP= 4 ATP Table 2: Inputs and Outputs of Citric Acid Cycle Citric Acid Cycle Inputs Outputs 1 1. 4 CO2 2 2. 6 NADH 3 3. 2 FADH2 4 4. 2 ATP Second Column Glycolysis-HEA= 2 NADH Prep-HEA= 2 NADH Krebs-HEA= 6 NADH and 2 FADH2 Third Column Glycolysis-ATP-OP= 4-6 ATP Prep-OP= 6 ATP Krebs-ATP-OP= 18 ATP and 4 ATP Table 3: ATP Harvest from Aerobic Respiration Teacher tip Total-ATP-OP=32-34 Phases in Aerobic Respiration Glycolysis Preparatory Reaction Krebs cycle ATP produced by Substrate-Level Phosphorylation High-energy Electron Acceptors ATP produced by Oxidative Phosphorylation Sub-total Fourth Column Glycolysis-S= 6-8 ATP Prep-S= 6 ATP Krebs-S= 24 ATP Total-S= 36-38 ATP -Table 4 Suggested Answers: Total Starting materials G: Glucose, ATP, NAD+, ADP, P F: Pyruvate, CoA, NAD+ K: Acetyl CoA, H2O, NAD+, FAD, ADP, P E: NADH, FADH2, O2, ADP, P -- Table 4: Starting Materials and End Products of Aerobic Respiration Stage Glycolysis (in cytosol) Formation of Acetyl CoA (in mitochondria) Krebs cycle (in mitochondria) Starting Materials End Products -- Electron Transport Chain and Chemiosmosis (in mitochondria -- Directions: As part of performance assessment, let the students do the 3-2-1 Closing. On their ½ crosswise paper, they write 3 things/concepts/key ideas they have learned in the lesson(s); 2 things/concepts/key ideas they have questions about the lesson(s); and 1 thing/concept/key idea they want the teacher to know about in connection to the lesson(s) discussed. 164 End Products G: Pyruvate, ATP, NADH F: Acetyl CoA, CO2, NADH K: CO2, NADH, FADH2, ATP E: ATP, H2O, NAD+, FAD General Biology 1 Energy Transformation Cellular Respiration (Part 3 of 3) Content Standard The learners demonstrate an understanding of cellular respiration. Performance Standard The leaners prepare simple fermentation setups using common fruits to produce wine or vinegar via microorganisms 240 MINS LESSON OUTLINE Introduction Discuss the nature of science with regard to cellular respiration. Raise several questions for the students to think about Motivation Instruction/ Let the students do activities on Delivery advantages and disadvantages of 1. Explain the advantages and disadvantages of fermentation and aerobic respiration (STEM_Bio11/12-IIa-j-12) Practice Answer the guide questions Enrichment Prepare materials for vinegar making Specific Learning Outcomes At the end of this lesson, the students must be able to: 1. Tabulate and explain the advantages and disadvantages of fermentation, anaerobic respiration and aerobic respiration; 2. Show the similarities and differences of fermentation, anaerobic respiration and aerobic respiration; 3. Compare the pathways of carbohydrate, fat, and protein catabolism. 5 15 aerobic and anaerobic respiration and fermentation together with their similarities and differences; prepare homemade virgin coconut oil and fermentation Learning Competency The learners: • Show a diagram of metabolic pool concept and ask few questions 15 Evaluation Compare and explain aerobic respiration, anaerobic respiration and fermentation, their advantage and disadvantage 160 5 40 Resources • • • • • Alumaga, Maria Jessica B. et al., (2014). Science and Technology 9. Quezon City: Vibal Publishing House Bawalan, Divina D. and Chapman, Keith R. (2006). Virgin Coconut Oil: Production Manual for Micro- and Village-scale Processing. Thailand: FAO Regional Office for Asia and the Pacific Mader, Sylvia S. (2010). Biology 10th Edition. USA: McGraw-Hill Rabago, Lilia M. et al., (2014). Science and Technology Laboratory Manual and Workbook for Grade 9 Quezon City: Vibal Group, Inc. Solomon, Eldra P. et al., (2008). Biology 8th Edition. China: Thomson Brooks/Cole INTRODUCTION (15 MINS) Communicate the learning competencies for this topic. You can enumerate some products (or show pictures) of fermentation. You may opt to insert and discuss the nature of science with regard to cellular respiration. For instance, athletes and trainers today are constantly finding ways to improve their performance in a particular event such as in triathlon by boosting cellular respiration. Why is meant by carbo-loading? Or as you go to the supermarket, you will see several kinds of energy drinks, are they safe to drink? What are the health implications if a person drinks them excessively? What about energy-boosting vitamins, are they really beneficial for the body? Or is it safe to drink an energy-boosting fluid rich in vitamin B-complex and other related substances when your stomach is empty? If you want to jump-start your mitochondria, would you rely on alternative medicine? These are interesting issues to discuss and bring to the whole class to think about. MOTIVATION (5 MINS) Show a metabolic pool concept to the class and ask the following questions: 1. What are the three kinds of food that provide the building blocks for the cells, and that all can provide energy? 2. What are the basic metabolic pathways organisms use to extract energy from carbohydrates in aerobic respiration? What about for proteins and fats? Are the pathways the same or not? 3. To which pathway do glycerol and fatty acids of fat enter? Suggested Answers: 1. Carbohydrates, proteins, and fats. 2. Glycolysis, Krebs cycle, electron transport chain—for carbohydrates, proteins and fats. The metabolic pathways for these three kinds of food are the same except that for proteins and fats, there are additional steps to get fats and proteins ready to enter the specific pathway as shown in the diagram. 3. Glycerol enters glycolysis; fatty acids enter preparatory reaction. 166 INSTRUCTION/DELIVERY (15 MINS) Directions: Tabulate and explain the advantages and disadvantages of fermentation, anaerobic respiration and aerobic respiration. Show their similarities and differences. Procedure: 1. Form five groups. Each group has an assigned topic to work on. The following groups are as follows: • GROUP 1: To work on the differences among aerobic, anaerobic and fermenting organisms. List all the possible answers as they can as long as the description written fits for the particular organism. • GROUP 2: To work on the similarities among aerobic, anaerobic and fermenting organisms. List all the possible answers as they can as long as the description written fits for the particular organism. • GROUP 3: To work on and explain the advantages and disadvantages of aerobic respiration. • GROUP 4: To work on and explain the advantages and disadvantages of anaerobic respiration. • GROUP 5: To work on and explain the advantages and disadvantages of fermentation. 2. Show to the class a sample table on how you want them to display, outline and report the information to the whole class. 3. Tell them to prepare Manila papers, marker, or any visual materials. Table 1: Activity: Differences and Similarities of Aerobic, Anaerobic and Fermenting Organisms Differences Aerobic Organisms Similarity Anaerobic Organisms Fermenting Organisms Aerobic, Anaerobic and Fermenting Organisms Table 2: Activity: Advantages and Disadvantages of Aerobic Respiration, Anaerobic Respiration and Fermentation Advantages of Aerobic Respiration Advantages of Anaerobic Respiration Advantages of Fermentation Disadvantages of Aerobic Organisms Disadvantages of Anaerobic Organisms Disadvantages of Fermenting Organisms Suggested Answers: Table 1: Activity: Differences and Similarities of Aerobic, Anaerobic and Fermenting Organisms Table 2: Activity: Advantages and Disadvantages of Aerobic Respiration, Anaerobic Respiration and Fermentation Differences Aerobic Organisms Similarity Anaerobic Organisms • Use oxygen. • Do not use oxygen. • H2O is the by-product. • • Electron acceptor is O2 and is reduced to water. H2O and potassium nitrite are the byproducts. • • With electron transport chain. With electron transport chain. • • Occur in prokaryotes and eukaryotes. Electron acceptor is nitrate or sulfate. • Occur in prokaryotes. • Requires no special organelles Fermenting Organisms • • • • • • • • Do not use oxygen. Lactate (lactate fermentation) or ethyl alcohol (alcoholic fermentation) is the byproduct. Final acceptors of electrons are pyruvate reduced to lactate, and acetaldehyde reduced to ethyl alcohol. No electron transport chain. Occur in prokaryotes and eukaryotes. Simple and faster alternative to cellular respiration Requires no special organelles Glycolysis and waste product formation are two sets of reactions that occur. 168 Aerobic, Anaerobic and Fermenting Organisms • ATP is produced. • CO2 is the waste product. • Electrons are transferred from glucose to NADH. Advantages of Aerobic Respiration • All available energy extracted from glucose is 36 to 38 ATP. • 39% energy transferred from glucose to ATP. Advantages of Anaerobic Respiration • All available energy extracted from glucose is 40 ATP (because prokaryotes have no mitochondria). • Slow breakdown of glucose into ATP. • • Organisms can do more work for a longer time with the slow and efficient breakdown of ATP. 43% energy transferred from glucose to ATP. • Complete breakdown of glucose. • Animals and the human muscle cells can adapt and perform lactic acid fermentation for a rapid burst of energy. • Can breathe heavily to refill the cells with oxygen so that lactate is removed from the muscle cells. • Lactate is returned to the liver to become pyruvate or glucose again. • Complete breakdown of glucose. Advantages of Fermentation • All available energy extracted from glucose is 2 ATP. • Certain bacteria produce chemicals of industrial importance such as isopropanol, butyric acid, acetic acid when bacteria ferment—breakdown of sugars in the absence of oxygen. • Foods that are fermented last longer because these fermenting organisms have removed many of the nutrients that would attract other microorganisms. • Yeasts ferment fruits and wine is produced. Grain is also fermented to produce beer. They also cause the bread to rise due to CO2, a by-product, and alcohol is lost in the bread. • Yeasts and lactobacillus together produce sour taste in wheat beer. • Yeasts and Acetobacter aceti spoil wine to become vinegar. • Bacterial fermentation produces yogurt (due to Streptococcus thermophilus and Lactobacillus bulgaricus), sour cream, cheese, brine cucumber pickles, sauerkraut, and kimchi. • Clostridium bacteria can produce nail polish remover and rubbing alcohol from the acetone and isopropanol they make • Soy sauce is produced by adding mold (Aspergillus), yeasts and fermenting bacteria. Disadvantages of Aerobic Organisms Disadvantages of Anaerobic Organisms Disadvantages of Fermenting Organisms • • • Consumption of 2 ATP is fast. • Ethanol and lactate, the by-products of fermentation, have a lot of energy reserves—prokaryotes and eukaryotes cannot extract the energy in lactate and ethanol using anaerobic method. • Needs a large supply of glucose to perform the same work as in aerobic respiration. • Glucose is partially oxidized. 61% of glucose metabolism becomes heat and enters the environment. • Human brain cells cannot perform lactic acid fermentation. • Human muscle cells feel the burning sensations and pain when lactate accumulates in the cell and experience oxygen debt. 57% of glucose metabolism becomes heat and enters the environment. Directions: Compare aerobic respiration, anaerobic respiration and fermentation in terms of the following factors listed in the first column. Activity Title: Comparison of Aerobic and Anaerobic Respiration and Fermentation Factors Aerobic Respiration Anaerobic Respiration Immediate fate of electrons in NADH Terminal electron acceptor of electron transport chain Reduced Product(s) formed Mechanism of ATP synthesis 170 Fermentation Suggested Answers: Factors Aerobic Respiration Anaerobic Respiration Fermentation Immediate fate of electrons in NADH Transferred to electron transport chain O2 Transferred to electron transport chain Transferred to organic molecule Inorganic substances such as NO3-- or SO4 2- No electron transport chain Water Relatively reduced inorganic substances Relatively reduced organic compounds (e.g., alcohol or lactate) Oxidative phosphorylation/ chemiosmosis; also substrate-level phosphorylation Oxidative phosphorylation/ chemiosmosis; also substrate-level phosphorylation Substrate-level phosphorylation only (during glycolysis) Terminal electron acceptor of electron transport chain Reduced Product(s) formed Mechanism of ATP synthesis Teacher Tip: Activity Title: Homemade Virgin Coconut Oil and Fermentation Materials needed: Fermentation containers (food-grade transparent plastics), basin or stainless stock pots, ladle (long spoon with deep bowl), cheesecloth (katsa), five fully matured nuts and coconut water, funnel, hand soap, water, cotton wool, glass bottle or PET bottle Procedures: • • 1. The natural fermentation method has two parts: (1) extraction and preparation of coconut milk and (2) processing of VCO from the milk The process for extracting, preparing and processing of VCO from the coconut milk are as follows: Fermentation refers to the addition of yeast or a specific microorganism or enzyme to a raw material to produce a desired product. But for the so-called natural fermentation method, we will produce VCO that does not require any addition of microorganisms or substance. When the coconut milk mixture is allowed to stand for at least 10 hours, the VCO will naturally separate from water and protein. Several theories say that the separation of these substances is due to the presence of airborne acetic acid bacteria (Acetobacter aceti). A. aceti breaks the protein bonds in the coconut milk causing the mixture to separate distinctively. 1. a. Selecting nuts — select fully five matured nuts (12 to 13 months) and de-husk; the husk should be turning brown. 2. Splitting and grating — split the de-husked nut into two manually and grate. Place the 3. First milk extraction — extract the milk from the grated coconut meat by hand using cheesecloth (katsa). Mix the grated coconut milk and the coconut water. Materials to be used such as fermentation containers, basin, ladle, and cheese cloth should be prepared neat and clean to avoid contamination. Wash your hands vigorously with water and soap.to kill and remove the presence of microorganisms that may alter the quality of VCO. Press the coconut meat thoroughly using your cheese cloth. Set aside the milk obtained using your clean fermentation container(s). Prepare the coconut residue (sapal) for the second round of milk extraction. 4. Second milk extraction — the ratio of mixing is 2 cups of milk residue (sapal) is to 1 cup of water coming from the first milk extraction. 5. Mixing of first and second milk extracts — mix well the first and second coconut milk extracts for 10 minutes. 6. Preparing for the fermentation containers — place the coconut milk extract in clean fermentation container(s). Cover the container(s) loosely as shown below. Place the container(s) in a place where temperature is 35 to 40oC. Allow the coconut milk mixture to settle for 16 to 24 hours for natural fermentation of the coconut milk extract to occur. 7. Separating the oil and fermented curd layers — separate the oil from the fermented curd by using ladle to scoop the oil off the top. Note: dispose of the water phase and gummy portion by diluting with water before draining into a grease trap. The fermented curd can be heated to remove the residual class B oil that can be used for making skin care and herbal soap products. The toasted curd can also be mixed with other compost material and use as organic fertilizer. 8. Filtering the oil — filter the VCO to remove adhering particles of fermented curd. 9. Packaging and storage — the recommended packaging material for VCO is glass. PET bottles can be used in cases where the VCO is immediately consumed. Note: Class A VCO is always waterclear. Class B VCO is yellow. The latter happens when the process of coconut milk extraction is invaded by unwanted microorganisms or sanitary protocols are not followed strictly such as washing the hands with antibacterial soap or washing the materials with antibacterial detergent soap. 10. Tell them to report their output to the class. 172 Another theory says that the enzyme present in the coconut makes the separation of substances to occur. The socalled ‘fermentation method’ happens when after 16 to 24 hours of settling, the water smells and tastes sour. The so-called ‘natural’ explains that there is no addition of any other substance or microorganism in fermenting the virgin coconut oil. Also the ‘virgin’ in the virgin coconut oil implies that there is no substance added to make the oil. Modified Natural Fermentation Method ENRICHMENT (90 MINS) Directions: Read the procedures below on how the jigsaw and expert PRACTICE (160 MINS) Procedure: Directions: 1. Explain why cells of most multicellular organisms cannot live long without oxygen. 2. How does poison like cyanide interfere with activity of electron transport chain in the mitochondrion? 3. Why is it beneficial for pyruvate to be reduced when oxygen in not available during the fermentation process? 4. If fermentation is a fast easy way to get ATP without oxygen and without requiring complex organelles, then why is there a slow more complex process involving oxygen? Suggested Answers: 1. The ATP produced during glycolysis is insufficient to sustain life processes. As a result, molecular oxygen has to appear to supply a bulk of ATP (almost 90%) to the body cells. And hence, most cells of multicellular organisms cannot live long without oxygen, especially the human brain cells which cannot undergo glycolysis. 2. Lack of oxygen is not the only factor that interferes with the electron transport system. Some poisons like cyanide inhibit the normal activity of the cytochrome found in the ETC. Cyanide binds tightly to the iron in the last cytochrome, making it unable to transport electrons to oxygen. This cyanide also blocks the passage of electron through the ETC. As this happens the production of ATP stops — and death ensues. 3. The reduction of pyruvate into lactate and alcohol ensures that NAD+ is regenerated, which is required for the first step in the energy-harvesting step of glycolysis. As NAD+ returns to the earlier reaction, it becomes reduced to NADH. In this way, glycolysis and substrate-level ATP synthesis continue to occur even without the presence of oxygen. 4. In aerobic respiration, the molecules are broken down slowly to get much more of that energy out and the left over products have useful energy left in them. Imagine a racing car were to get instantly all the energy from fuel. If this happened, the racing car could not run longer mileage. ENRICHMENT (5 MINS) Teacher Tip: This can be done at home as group assignment. Just give your students precautionary measures. Instructions can be given for five minutes. Tell them to document their output and submit it via YouTube or Facebook. Activity Title: Vinegar Making Materials needed: 9 cups of coconut water, 2 cups of brown sugar, ½ teaspoon yeast, 2 clean cheesecloth, transparent bottles for transferring the mixture, gas stove, rubber bands, cooking pan, funnel, 2 cups of mother vinegar, jar(s) spoon for mixing Procedure: SET A: Preparation and alcoholic fermentation 1. Using cheesecloth, filter the coconut water and place it a clean cooking pan. Then add 2 cups of brown sugar. Mix thoroughly using a spoon until the sugar crystals are dissolved completely. 2. Heat the mixture at low fire for 20 minutes. As you do this, do not cover the cooking pan and do not boil the mixture. After 20 minutes, let the mixture cool for 30 minutes to 1 hour. 3. Add ½ teaspoon of yeast. Mix very well. Afterwards, transfer the mixture to clean transparent 174 Vinegar is a sour-tasting condiment and preservative. It can be prepared by two successive microbial processes. The first phase is done through alcoholic fermentation by a eukaryotic organism called yeast. The second phase is by oxidation of alcohol by a prokaryotic organism called Acetobacter aceti. This bacterium is responsible for converting the alcohol in wine to acetic acid or vinegar. Since coconut is abundant in our country, use this example to show the principle of fermentation process involving microorganisms and the series of reactions that take place as coconut water is converted into vinegar. 4. bottle(s). Cover the bottle(s) with cheesecloth. The small pores in the cheesecloth will allow the gas from inside the bottle to exit. 5. Place the mixture in a safe place to allow the process of fermentation. Note: From the day you added yeasts to the mixture you will observe alcoholic fermentation that is characterized by the release of bubbles. The bubbles indicate the presence of carbon dioxide. When bubbles no longer appear in the mixture, then alcoholic fermentation has ceased already. The formation of bubbles will be observed for approximately one week. To ferment means to break the sugar in the absence of oxygen. SET B: Acetous Fermentation After one week, transfer the mixture to a jar. Then add 2 cups of mother vinegar to a jar. Cover the jar with cheesecloth to allow oxygen to enter into the jar. Place the jar in a safe place. Wait for one month to allow acetous fermentation. EVALUATION (40 MINS) Directions: Compare and explain aerobic respiration, anaerobic respiration and fermentation in terms of the following: 1. Immediate fate of electron transfer 2. Terminal electron acceptor of electron transport chain 3. Reduced product(s) formed 4. Mechanism of ATP synthesis Tabulate your answers. Then give at least three advantages and at one disadvantage for aerobic respiration, anaerobic respiration and fermentation. General Biology 1 120 MINS ATP in Cellular Metabolism and Photosynthesis LESSON OUTLINE Content Standards The learners demonstrate an understanding of: Introduction Review of prerequisite and related topics 1. ATP-ADP Cycle 2. Photosynthesis Motivation 3. Respiration Performance Standard The learners shall be able to prepare simple fermentation setup using common fruits to produce wine or vinegar via microorganisms. Learning Competencies • The learners describe reactions that produce and consume ATP (STEM_BIO11/12-IIa-j-9) • The learners compute the number of ATPs needed or gained in photosynthesis and respiration (STEM_BIO11/12_IIa-j-11) Specific Learning Outcome At the end of the lesson, the learners shall be able to compute the number of ATPs needed or gained in photosynthesis and respiration 176 Engage students with questions of purpose 30 5 Instruction/ Lecture on Photosynthesis, ATP Delivery/ Production, and Cellular Metabolism Practice 60 Practice Group Activity 15 Evaluation Quiz 10 Resources Shown/presented on the different parts of the Teaching Guide INTRODUCTION (30 MINS) Clearly communicate learning competencies and objectives. At the end of the session, the learners shall be able to compute the number of ATPs needed or gained in photosynthesis and respiration. Also, allow the readers to connect and/or review prerequisite knowledge. Review structure of ATP (Adenosine Triphosphate) and how it is used as the energy currency of the cell. During PHOTOSYNTHESIS: • Energy from sunlight is harvested and used to drive the synthesis of glucose from CO2 and H2O. By converting the energy of sunlight to a usable form of potential chemical energy, photosynthesis is the ultimate source of metabolic energy for all biological systems. • Photosynthesis takes place in two distinct stages. (A) In the light reactions, energy from sunlight drives the synthesis of ATP and NADPH, coupled to the formation of O2 from H2O. (B) In the dark reactions (named because they do not require sunlight), the ATP and NADPH produced by the light reactions drive glucose synthesis. • In eukaryotic cells, both the light and dark reactions of photosynthesis occur within chloroplasts—the light reactions in the thylakoid membrane and the dark reactions within the stroma. Stages of Cellular Metabolism: 1. Glycosis 2. Pyruvate grooming (between Glycolysis and Citric acid cycle 3. Kreb’s Cycle/Citric Acid Cycle/Acid Cycle 4. Electron Transport Chain and Oxidality Compare and Contrast Cellular Respiration and Photosynthesis Cellular Respiration Photosynthesis Production of ATP Yes; theoretical yield is 38 ATP molecules per glucose but actual yield is only about 30-32. Yes Reactants C6H12O6 and 6O2 6O2 and 12H2O and light energy Requirement of sunlight Sunlight not required; cellular respiration occurs at all times. Can occur only in presence of sunlight Chemical Equation (formula) 6O2 + C6H12O6 --> 6CO2 +6H2O + ATP (energy) 6CO2 + 12H2O + light --> C6H12O6 + 6O2 + 6H20 Process Production of ATP via oxidation of organic sugar compounds. [1] glycolosis: breaking down of sugars; occurs in cytoplasm [2] Krebs Cycle: occurs in mitochondria; requires energy [3] Electron Transport Chain-- in mitochondria; converts O2 to water. The production of organic carbon (glucose and starch) from inorganic carbon (carbon dioxide) with the use of ATP and NADPH produced in the light dependent reaction Fate of oxygen and carbon dioxide Oxygen is absorbed and carbon dioxide is released. Carbon dioxide is absorbed and oxygen is released. Energy required or released? Releases energy in a step wise manner as ATP molecules Requires energy Main function Breakdown of food. Energy release. Production of food. Energy Capture. Chemical reaction Glucose is broken down into water and carbon dioxide (and energy). Carbon dioxide and water combine in presence of sunlight to produce glucose and oxygen. Stages 4 stages: Glycolysis, Linking Reaction (pyruvate oxidation), Krebs cycle, Electron Transport Chain (oxidative phosphorylation). 2 stages: The light dependent reaction, light independent reaction. (AKA light cycle & calvin cycle) What powers ATP synthase H+ proton gradient across the inner mitochondria membrane into matrix. High H+ concentration in the intermembrane space. H+ gradient across thylakoid membrane into stroma. High H+ concentration in the thylakoid lumen Products 6CO2 and 6H20 and energy(ATP) C6H12O6 (or G3P) and 6O2 and 6H2O Cellular Respiration Photosynthesis What pumps protons across the membrane Electron transport chain. Electrochemical gradient creates energy that the protons use to flow passively synthesizing ATP. Electron transport chain Occurs in which organelle? Mitochondria Glycolysis (cytoplasm) Chloroplasts Final electron receptor O2 (Oxygen gas) NADP+ (forms NADPH ) Occurs in which organisms? Occurs in all living organisms (plants and animals). Occurs in plants, protista (algae), and some bacteria. Electron source Glucose, NADH + , FADH2 Oxidation H2O at PSII Catalyst - A substance that increases the rate of No catalyst is required for respiration reaction. a chemical reaction Reaction takes places in presence of chlorophyll. High electron potential energy From light photons. From breaking bonds Photosynthesis (source: http://www.diffen.com/difference/Cellular_Respiration_vs_Photosynthesis) MOTIVATION (5 MINS) Teacher asks students “Why study photosynthesis and cellular respiration?” Photosynthesis - Lead discussion into the importance of 1. Photosynthesis in selection of plants/trees for reforestation, agriculture and biodiversity conservation. (http://bioenergy.asu.edu/photosyn/ study.html) 2. Cellular Respiration in food production (pretzels, beer, soy sauce, pickles, vinegar, yeast-risen bread, or any other product that requires fermentation or microbial breakdown of compounds in food), athletics (sports nutrition and event-specific training) and others. INSTRUCTION/DELIVERY/PRACTICE (60 MINS) Lecture on Photosynthesis and ATP Production ATP is formed by the addition of a phosphate group to a molecule of adenosine diphosphate (ADP); or to state it in chemical terms, by the phosphorylation of ADP. This reaction requires a substantial input of energy, much of which is captured in the bond that links the added phosphate group to ADP. Because light energy powers this reaction in the chloroplasts, the production of ATP during photosynthesis is referred to as photophosphorylation. The light reaction uses the energy from photons to create ATP and NADPH, which are both forms of chemical energy used in the dark reaction (=Calvin Cycle). Calvin Cycle, through a series of chemical reactions, takes the carbon from carbon dioxide and turns it into glucose, which is a sugar that cells use as energy. Carbon dioxide is a fully oxidized molecule. What that means is basically it does not have a lot of chemical energy. The Calvin Cycle turns carbon dioxide into the much more reduced molecule, glucose, which has much more energy. Where ATP comes into play is that it functions as a reduced molecule that provides the chemical energy that transforms the carbons in the carbon dioxide molecules into progressively more reduced molecules until you get glucose. Thus, ATP is used to power the change from 3phosphoglycerate to 1,3-bisphosphoglycerate, which is then turned into 2 glyceraldehyde 3-phosphate (G3P) molecules with NADPH (an energy source similar to ATP). One of these G3P molecules gets turned into glucose. The other one is transformed by ATP into ribulose bisphosphate (RuBP), which then picks up carbon dioxide and the cycle begins again. Lecture on Cellular Metabolism and ATP Production What is the difference between substrate-level phosphorylation and oxidative phosphorylation? Substrate-level phosphorylation – is the formation of ATP by the direct transfer of a PO3 group to ADP. (Source: https://quizlet.com/11951424/metabolism-final-exam-flash-cards/) Oxidative phosphorylation – is the process that explains how molecules of FADH2 and NADH are used to make ATP. The term “oxidative” is used because oxygen accepts an electron while the gradient made by the movement of electrons powers the creation ATP. (Source: http://www.dbriers.com/tutorials/2012/04/substrate-level-vs-oxidative-phosphorylation/) Other references that can be used: https://online.science.psu.edu/biol110_sandbox_8862/node/8924 http://www.neshaminy.k12.pa.us/Page/20741 180 Review Stages of Cellular Metabolism and the products produced at each stage (ATP by substrate level phosphorylation, CO2, NADH and FADH2) Four Major Reaction Pathways 1. Glycolysis 2. Conversion of Pyruvate to Acetyl CoA (also called Oxidation of Pyruvate, Pyruvate Processing, Pyruvate Grooming) 3. Kreb’s Cycle (Citric Acid Cycle, Tricarboxylic Acid Cycle) 4. Electron Transport Chain (Chemiosmosis) Other references that can be used: • • • • • • • • • • • https://courses.candelalearning.com/ap2x1/chapter/carbohydrate-metabolism/ http://www.uic.edu/classes/bios/bios100/lectures/respiration.htm http://www.neshaminy.k12.pa.us/Page/20741 http://sp.uconn.edu/~bi102vc/1102fall11/ATP.html http://biology.tutorvista.com/cell/cellular-respiration.html https://en.wikibooks.org/wiki/Biology,_Answering_the_Big_Questions_of_Life/Metabolism/Metabolism3 http://people.ucalgary.ca/~rosenber/CellularRespirationSummary.html http://antranik.org/intro-to-cellular-respiration-the-production-of-atp/ http://sandwalk.blogspot.com/2007/12/how-cells-make-atp-substrate-level.html http://study.com/academy/lesson/substrate-level-phosphorylation-and-oxidative-phosphorylation.html http://www.diffen.com/difference/Cellular_Respiration_vs_Photosynthesis Stage of Cellular Metabolism Substrate-Level Phosphorylation Oxidative Phosphorylation through ETC NADH Subtotal1 Glycolysis 2 2 4-6 2 Pyruvate Grooming (Decarboxylation of Pyruvate)(x2)3 0 2 6 Citric acid cycle ( x 2)3 2 6 18 Subtotal 4 28-30 FADH2 Subtotal1 2 4 4 Total 36-38 SUMMARY TABLE – Number of ATP molecules formed from 1 molecule of glucose The amount of ATP produced is estimated from the number of protons than passes through the inner mitochondrial membrane (via the electron acceptors of the electron transport chain (ETC) and the number of ATP produced by ATP Synthase. 1. Assumption = Each NADH will generate 3 ATPs while FADHs will generate 2 ATPs. 2. The number of ATP produced depends on the acceptor that receives the hydrogen ions and electrons from the NADH formed during glycolysis in the cytoplasm. 3. Glycolysis results in formation of 2 molecules of pyruvic acid/pyruvate thus values are multiplied by 2. STAGE LOCATION WHAT REACTANT (What PRODUCTS (What ATP PRODUCED BY HAPPENS? goes in) comes out) Substrate Level Phosphorylation Oxidative Phosphorylation NADH FADH2 Glycolysis Pyruvate Processing/ Grooming Citric Acid Cycle Oxidative Phosphorylation PRACTICE (15 MINS) Using previous lessons and this lecture, students grouped into 2-3 are asked to answer the table below. Teacher provides the initial number of glucose molecules that will undergo cellular metabolism. Practice Questions (Available at the following sites: Students are asked to watch a video and answer questions after watching the video). Photosynthetic Electron Transport and ATP Synthesis • https://highered.mheducation.com/sites/9834092339/student_view0/chapter39/photosynthetic_electron_transport_and_atp_synthesis.html 182 Calvin Cycle • https://highered.mheducation.com/sites/9834092339/student_view0/chapter39/calvin_cycle.html EVALUATION (10 MINS) Questions for Exam/Quiz • https://quizlet.com/11951424/metabolism-final-exam-flash-cards/ • https://quizlet.com/17507853/biochemistry-ii-practice-questions-oxidative-phosphorylation-flash-cards/ • http://faculty.une.edu/com/courses/bionut/distbio/obj-512/Chap21-practice%20questions.htm • http://web.mnstate.edu/provost/Chem410ETSOxPhosQuest.pdf • https://www.khanacademy.org/test-prep/mcat/biomolecules/krebs-citric-acid-cycle-and-oxidative-phosphorylation/e/oxidativephosphorylation-questions • https://mcb.berkeley.edu/labs/krantz/mcb102/MCB102-SPRING2008-EXAM-KEY_v3.pdf RUBRICS/ASSESSMENT GUIDE LEARNING COMPETENCY ASSESSMENT TOOL Exemplary (8-10) Satisfactory (5-7) Developing (3-4) The learners shall be able to describe the following: Student participation (During lecture) Student was able to answer the question without referring to his/her notes plus the follow-up question. Student was able to answer the question without referring to his/ her notes; Was not able to answer follow up question. Student was able to (1) Student was not able answer the question but to answer the question. read from his/her notes. (2) Student read from notes of his/her classmate.. Students in the team equally contributed to the discussion and the answering of the table provided Student listened to the discussion but contribution to the team was lesser than the other members Student was a passive participant and contribution was minimal. Student was interested in other matters not related to the exercise. Obtained 50-69.99% correct answers in the exam Obtained percentile <50% correct answers in the exam Compute the number of ATPs needed or gained in Student participation photosynthesis and (During Practice) respiration Examination Obtained 90-100% correct Obtained 70-80.99% answers in the exam correct answers in the exam Beginning (1-2) Biographical Notes FLORENCIA G. CLAVERIA, Ph.D. Team Leader DAWN T. CRISOLOGO Team Leader Dr. Florencia G. Claveria is the current Chair of the CHED Technical Panel for Biology and Molecular Biology. She is also member of the Commission’s Technical Panel for Math and Science. She is currently Vice Chancellor for Academics, Research, and Operations at the De La Salle Araneta University. She is a full professor at the De La Salle University-Manila where she served as Dean of the College of Science for 6 academic years. Dr Claveria finished her doctorate in Biological Sciences at the University of Cincinnati, through a Fulbright-Hays grant. She completed her master’s in Zoology at the Ghen State University, through a grant from the Government of Belgium. She earned her bachelor’s degree in Biology at St. Louis University. Her written scholarly works include contributions to academic publications such as the Philippine Textbook of Medical Parasitology, Journal of Protozoology Research, and The Journal of Veterinary Medical Science. 184 Ms. Dawn Crisologo is a Special Science Teacher at the Philippine Science High School-Main Campus in Diliman, Quezon City and specializes in advanced topics in Ecology, Evolution and Biodiversity, Anatomy, Physiology, and Methods in Science and Technology Research. She is a member of the Asian Association of Biology Educators, Wildlife Conservation Society of the Philippines, and Biology Teachers Association of the Philippines. Her works are included in The Philippine BIOTA Journal and three editions of the Science Blast textbook. Ms. Crisologo is currently finishing her master’s in Environmental Science at the University of the Philippines Diliman. She completed her bachelor’s degree in Biology at the same university. CHUCKIE FER CALSADO Writer Mr. Chuckie Fer Calsado is Special Science Teacher IV at the Philippine Science High School Main Campus where he has been teaching for 8 years. He is a member of biological organisations like the Biology Teachers Association of the Philippines, the Asian Association for Biology Education, and Concerned Artists of the Philippines among many others. He has published academic papers such as Implication of Students’ Cognitive Style, Personal Demographics, Values and Decision Making in Environmental Education and the Role of Education in the Prevention of Child Trafficking in Nepal. Mr. Calsado finished his Master’s in Bioethics at the Monash University and his bachelor’s degree in Biology at the University of the Philippines DIliman. AILEEN C. DELA CRUZ Writer JANET S. ESTACION, Ph.D. Writer Ms. Aileen Dela Cruz has been serving as the Science Research Analyst at the Philippine Science High School - Main Campus since 2004. Her academic interests range from microbiology, food safety and nutrition, and laboratory safety and she has been involved in trainings and conferences on the same fields of study. Her published scholarly works include series of textbooks on 21st Century Learning. Ms. Dela Cruz earned her bachelor’s degree in Biology at the University of the Philippines Baguio. Dr. Janet Estacion is current Officer-in-Charge at the Institute of Marine and Environmental Science in Silliman Unive DOREEN D. DOMINGO, PH.D. Writer Dr. Doreen D. Domingo is a Professor at the Mariano Marcos State University where she teaches both in the graduate and undergraduate levels. She is currently the Chief of Alumni Relations for the university. Dr. Domingo finished her doctorate in Biology (magna cum laude) at St. Louis University through a research grant from CHED and the Microbial Forensics and Biodefense Laboratory, Indiana University. She completed her Doctor of Education on Educational Management, her master’s degree in Education major in Biology, and her bachelor’s degree in Biology at the Mariano Marcos State University. Dr. Domingo’s scholarly works were published on the International Referred Journal and the National Referred Journal. rsity where she has been teaching for 30 years now. She headed researches on marine conservation and the recovery of reefs. Her scholarly works appeared on different publications such as the Philippine Science Letters and the Silliman Journal. Dr. Estacion earned her doctorate degree in Zoology at the James Cook University of North Queensland. She completed her master’s degree in Marine Biology at the University of the Philippines Diliman and her bachelor’s degree in Biology at the Silliman University. MARY JANE C. FLORES, Ph.D. Writer Dr. Mary Jane C. Flores is Assistant Professor 3 at the College of Science in the De La Salle University where she has been teaching for 20 years now. Her published works include researches on parasitology, climatology, and community nutrition. Dr Flores has conducted and attended seminars on Biology in the country and abroad, including the Training on Biological Control at the US Department of AgricultureAgricultural Research Service and Congress meetings on Parasitology. She is a two-time recipient of the Don Ramon J. Araneta Chair in Ecology among other citations. Dr. Flores earned her Doctorate, Master’s, and Bachelor’s degrees in Biology at the De La Salle University. JOHN DONNIE RAMOS, Ph.D. Technical Editor JUSTIN RAY M. GUCE Writer Mr. Justin Ray Guce is a Special Science Teacher I at the Philippine Science High School Main Campus in DIliman, Quezon City where he teaches for 9 years. He has served as a Trainer of student representatives for Science Olympiad competitions and has delivered presentations in a number of Biology workshops and conventions. Mr Guce is a member of the Wildlife Conservation Society of the Philippines and the Biology Teachers Association of the Philippines. Mr Guce is currently finishing his master’s in Biology Education at the University of the Philippines Diliman where he also graduated his bachelor’s degree in Biology. NOLASCO H. SABLAN Writer Mr. Nolasco Sablan is Teacher III at the Parada National High School and is a DepEd teacher for 11 years now. He has worked as resource speaker, trainer, and writer for different institutions in the education sector, including the Ateneo de Manila University, Metrobank Foundation Inc., and the Department of Education. Mr. Nolasco Sablan earned his master’s degree in Biology Education at the Ateneo de Manila University and completed his bachelor’s degree in Education major in General Science at the Philippine Normal University. 186 Dr. John Donnie Ramos is a Member of CHED’s Technical Panel for Biology and Microbiology and Board Member of the Philippine Society for Biochemistry and Molecular Biology. He is currently the Dean of the College of Science at the University of Santo Tomas where he teaches molecular biology, immunology and genetics, and allergology. Dr. Ramos completed his doctorate in Molecular Biology at the National University of Singapore. He finished his master’s degree in Biological Sciences at the University of Santo Tomas and his bachelor’s degree in Biology at the Philippine Normal University. Dr. Ramos is recipient of the NAST-TWAS Prize for Young Scientist in the Philippines in 2010, and Outstanding Young Scientist by the National Academy of Science and Technology in 2005. JOY R. JIMENA Copyreader Ms. Joy Jimena is currently Planning Officer II at the Information Management Bureau of the Department of Social Welfare and Development. She also previously worked with other government agencies such as the Department of National Defense and Philippine Commission on Women, and Social Security System. Ms. Jimena graduated at the University of the Philippines Diliman with a degree in Public Administration. RENAN U. ORTIZ Illustrator Mr. Renan Ortiz is a teacher and visual artist who has collaborated in local and international art exhibitions such as the SENSORIUM at the Ayala Museum, Populus in Singapore, Censorship_2013 Move On Asia in South Korea, and the Triumph of Philippine Art in New Jersey, USA. Mr. Ortiz’s solo exhibitions include versereverse at the Republikha Art Gallery. He first completed his bachelor’s degree in Political Science at the University of the Philippines Manila before finishing his bachelor’s degree in Fine Arts major in Painting at the University of the Philippines Diliman. Mr. Ortiz is an awardee of the Cultural Center of the Philippines’ CCP Thirteen Artists Awards in 2012. DANIELA LOUISE B. GO Illustrator Ms Daniela Louise Go is a freelance illustrator and graphic designer, specializing on graphic design, brand and campaign design, and copywriting. She has worked as illustrator for Stache Magazine, Philippine Daily Inquirer, and Summit Media Digital. Ms Go is a member of organisations such as the UP Graphic and UP Grail in which she also served as designer and illustrator. Her works have been part of art exhibitions including Freshly Brewed, Wanton Hypermaterialism, and Syntheses 2014: Graduate Exhibit. Ms. Go graduated her bachelor’s degree in Fine Arts Major in Visual Communication at the University of the Philippine Diliman.