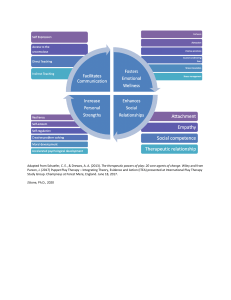
Clicker Questions Chapter 9 Substitution and Elimination Reactions of Alkyl Halides © 2017 Pearson Education, Inc. What happens in the following SN2 reaction if the concentration of both reagents is doubled? A. The reaction rate doubles. B. The reaction rate triples. C. The reaction rate is quadrupled. D. The reaction rate is unaffected. E. The reaction rate is halved. © 2017 Pearson Education, Inc. What happens in the following SN2 reaction if the concentration of both reagents is doubled? A. The reaction rate doubles. B. The reaction rate triples. C. The reaction rate is quadrupled. D. The reaction rate is unaffected. E. The reaction rate is halved. rate = k2[halide][nucleophile] © 2017 Pearson Education, Inc. Which of the following factors favor an SN2 reaction with a negatively charged nucleophile? A. a good leaving group B. a good nucleophile C. an unhindered reaction center D. an aprotic polar solvent E. all of the above © 2017 Pearson Education, Inc. Which of the following factors favor an SN2 reaction with a negatively charged nucleophile? A. a good leaving group B. a good nucleophile C. an unhindered reaction center D. an aprotic polar solvent E. all of the above The rate of an SN2 reaction is dependent upon all of these factors. © 2017 Pearson Education, Inc. Which of the following species is the best nucleophile in methanol? A. F− B. NH3 C. CH3S− D. CH3O− E. HO− © 2017 Pearson Education, Inc. Which of the following species is the best nucleophile in methanol? A. F− B. NH3 C. CH3S− D. CH3O− E. HO− © 2017 Pearson Education, Inc. In a protic solvent, nucleophilicity increases down a column. Which of the following substrates react fastest in an SN2 reaction? A. vinyl bromide B. cyclohexyl bromide C. phenyl iodide D. 2-bromopropane E. benzyl bromide © 2017 Pearson Education, Inc. Which of the following substrates react fastest in an SN2 reaction? A. vinyl bromide B. cyclohexyl bromide C. phenyl iodide D. 2-bromopropane E. benzyl bromide © 2017 Pearson Education, Inc. The alkyl halide is primary. Vinyl bromide and phenyl iodide do not undergo SN2 reactions Which of the following best explains a partial racemization? A. a good leaving group B. an SN2 reaction C. formation of an intimate ion pair D. completely dissociated ions E. a polar solvent © 2017 Pearson Education, Inc. Which of the following best explains a partial racemization? A. a good leaving group B. an SN2 reaction C. formation of an intimate ion pair D. completely dissociated ions E. a polar solvent The leaving group must be fully dissociated for complete racemization to occur in an SN1 reaction. © 2017 Pearson Education, Inc. Which of the following statements is not true about the SN1 reaction of an alkyl halide? A. Tertiary alkyl halides and benzyl and allyl halides undergo SN1 reactions. B. The rate is dependent upon the stability of the carbocation. C. The rate is dependent upon the concentration of the nucleophile. D. The reaction requires a good leaving group. E. The reaction is favored in a polar protic solvent. © 2017 Pearson Education, Inc. Which of the following statements is not true about the SN1 reaction of an alkyl halide? A. Tertiary alkyl halides and benzyl and allyl halides undergo SN1 reactions. B. The rate is dependent upon the stability of the carbocation. C. The rate is dependent upon the concentration of the nucleophile. D. The reaction requires a good leaving group. E. The reaction is favored in a polar protic solvent. The rate-determining step is formation of the carbocation, which is independent of the nucleophile. © 2017 Pearson Education, Inc. Which of the following statements is false? A. Increasing solvent polarity decreases the reaction rate when any reactant is charged. B. Increasing solvent polarity increases the reaction rate when none of the reactants is charged. C. An E2 reaction is favored by a high concentration of a strong, hindered base. D. An E1 reaction is favored by a secondary alkyl halide. E. An E2 is favored by a strong base. © 2017 Pearson Education, Inc. Which of the following statements is false? A. Increasing solvent polarity decreases the reaction rate when any reactant is charged. B. Increasing solvent polarity increases the reaction rate when none of the reactants is charged. C. An E2 reaction is favored by a high concentration of a strong, hindered base. D. An E1 reaction is favored by a secondary alkyl halide. E. An E2 is favored by a strong base. Secondary alkyl halides do not undergo SN1/E1 solvolysis reactions. © 2017 Pearson Education, Inc. Which of the following compounds undergoes an E2 reaction most readily? A. © 2017 Pearson Education, Inc. B. C. D. E. Which of the following compounds undergoes an E2 reaction most readily? A. B. C. D. E. Only in D are both substituents to be eliminated in axial positions. © 2017 Pearson Education, Inc. Which of the following bases most favors elimination over substitution? H2O A. © 2017 Pearson Education, Inc. CH3O− (CH3)3CO− CN− B. C. D. CH3S− E. Which of the following bases most favors elimination over substitution? H2O A. CH3O− (CH3)3CO− CN− B. C. D. CH3S− E. A strong, bulky base favors elimination over substitution. © 2017 Pearson Education, Inc. Which alkyl halide reacts the fastest with sodium methoxide under E2 conditions? © 2017 Pearson Education, Inc. A. B. D. E. C. Which alkyl halide reacts the fastest with sodium methoxide under E2 conditions? A. D. © 2017 Pearson Education, Inc. B. E. C. I− is the best leaving group, and E has eight hydrogens that can readily be removed by a strong base. In the following reaction, which base most favors the anti-Zaitsev product? A. © 2017 Pearson Education, Inc. B. C. D. E. In the following reaction, which base most favors the anti-Zaitsev product? A. B. C. D. E. The most sterically hindered base favors anti-Zaitsev elimination. © 2017 Pearson Education, Inc.