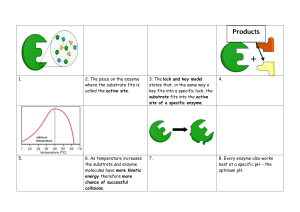
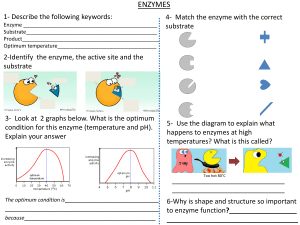
Factors that affect enzyme action. The effect of enzyme concentration. Enzymes contain active sites in which the substrates fit to be broken down to products. The greater the concentration of enzyme, the faster the rate of reaction because they are more frequents the collisions between enzyme and substrate due to more active sites. Initially, the rate of reaction increase steeply with increasing concentration of enzyme. This is because there are more active sites for the substrates to slot in and be broken down into products. The rate of reaction gradually increases with increase in enzyme concentration because the substrate concentration is reducing due to many active sites. Thus the substrate concentration becomes a limiting factor. The effect of substrate concentration. The higher the concentration of the substrate, the faster the rate of reaction because more substrates are available to be broken down into products due high collisions between the substrates and enzymes. As substrates concentration increases, the initial rate of reaction also increases because there are more substrates which increase the rate collision with the enzymes` active sites. If the substrate concentration is increased but that of enzyme kept constant, there comes a point where every enzyme active site is working continuously. The enzyme simply cannot work faster thus substrate molecules have to queue up to wait for the active site become vacant. The point at which the enzyme is working at its maximum possible rate is known as Vmax. Or. With further increase in substrate concentration, the rate becomes constant because there are few active sites and so substrate have to wait for active sites to be free so that they are worked on. But if at this point the enzyme concentration is increased, the rate of reaction increases steeply. Temperature and enzyme activity At low temperatures, the reaction takes of place only very slowly. This is because molecules are moving relatively slowly. Substrate molecules will not often collide with the active site, and so binding between substrate and enzyme is rare. As temperature rises, the enzyme and substrate molecules move faster. Collisions happen more frequently, so that substrate molecules enter the active site more often increasing the rate of reaction. As temperature continues to increase, the speed of movement of the substrate and enzyme molecules also continues to increase. Above the optimum temperature, the structure of the enzyme molecule vibrates so energetically that some of the bonds (hydrogen bonds) holding the enzyme molecule in its precise shape begins to break. The enzyme molecule begins to lose its shape and activity, and is said to be DENATURED. The substrate molecule fits less well into the active site of the enzyme, so the rate of the reaction begins to slow down and eventually the substrate no longer fits at all and the rate becomes zero. Optimum temperature- temperature at which an enzyme catalyses a reaction at the maximum rate. Most human enzymes have optimum temperature around 400C. Enzymes form other organisms have different optimum temperatures. PH. Most enzymes work fastest at a pH of fairly neutral conditions. Some such as the protease pepsin work in acidic conditions and have a different optimum pH. The lower the pH, the higher the hydrogen ion concentration. These ions can interact with R groups of amino acids affecting the charges of the groups. This affects the ionic bonding between the groups which in turn affects the threedimensional arrangement of the enzyme molecule. The shape of the active site may change and therefore reduce the chances of the substrate molecule fitting into it. A pH which is very different form the optimum pH can cause denaturation of an enzyme. ENZYME INHIBITORS Competitive, reversible inhibition. If some other molecule has a very similar shape as the active site, this will bind to an enzyme`s active site and inhibit the enzyme`s function. When the inhibitor binds briefly to the active site, there is competition between it and the substrate for the site. In competitive inhibition, the inhibitor has the same shape as the active site and so both the inhibitor and substrate compete for the same active site. The rate of enzyme active will slow down since few substrates are able to collide with the active site. If there is much more of the substrate present than the inhibitor, the substrate molecules can easily bind to the active site in the usual way and so the enzyme`s function is unaffected. If the concentration of the inhibitor rises, or that of the substrate falls, it becomes less and less likely that the substrate will collide with an empty site. Competitive inhibition is said to be reversible because it can be reversed by increasing the concentration of the substrate. Non-competitive inhibition A molecule can bind to another part of the enzyme rather than the active site. While the inhibitor is bound to the enzyme it can seriously disrupt the normal arrangement of hydrogen bonds and hydrophobic interactions holding the enzyme molecule in its three-dimensional shape. The resulting distortion ripples across the molecule to the active site making the enzyme unsuitable for the substrate. Thus the enzymes action is blocked no matter how much substrate is present. Inhibition of enzyme function may be lethal but in many situations it is essential. For example metabolic reactions must be very finely controlled and balanced, so no single enzyme can be allowed to run wild leading to more and more products. One way of controlling metabolic reactions is to use the end-product of a chain of reactions as a non-competitive, reversible inhibitor. As the enzyme converts substrate to product, it is slowed down because the end-product binds to another part of the enzyme and prevents more substrate binding. However, the end-product can lose its attachment to the enzyme and go to be used elsewhere, allowing the enzyme reform into its active state. As product levels fall, the enzyme is able to top them up again. This is termed end-product inhibition. As levels of product 3 rise, there is increasing inhibition of enzyme 1. So, less product 1 is made and hence less product 2 and 3. Falling levels of the product 3 allow increased function of enzyme 1 so products 1, 2 and3 rise again and the cycle continues. This end-product inhibition finely controls levels of product 3 between narrow upper and lower limits and is an example of a feedback mechanism.