Scientia Horticulturae 246 (2019) 347–353
Contents lists available at ScienceDirect
Scientia Horticulturae
journal homepage: www.elsevier.com/locate/scihorti
Spring late frost resistance of selected wild apricot genotypes (Prunus
armeniaca L.) from Cappadocia region, Turkey
T
Hatice Dumanoglua, Veli Erdogana, , Ali Kesika, Said Efe Dostb, Rabia Albayrak Delialiogluc,
Zahide Kocabasc, Cemil Ernimd, Tahir Macite, Melike Bakirf
⁎
a
Ankara University, Faculty of Agriculture, Department of Horticulture, Ankara, Turkey
Karamanoğlu Mehmetbey University, Vocational School of Technical Sciences, Karaman, Turkey
c
Ankara University, Faculty of Agriculture, Biometry and Genetics Unit, Ankara, Turkey
d
Apricot Research Institute, Malatya, Turkey
e
Malatya Directorate of Provincial Agriculture and Forestry, Malatya, Turkey
f
Erciyes University, Seyrani Faculty of Agriculture, Department of Agricultural Biotechnology, Kayseri, Turkey
b
ARTICLE INFO
ABSTRACT
Keywords:
Wild apricot
Genetic resources
Frost damage
Freezing tests
Pistil and seed viability
Electrical conductivity
Spring late frost is the most important problem in apricot growing. One of the effective and long-lasting solutions
is the development of genotypes with frost resistance as well as late flowering. In this study, low temperature
resistance of 36 wild apricots (Prunus armeniaca L.), selected for their survival after severe spring late frosts in
their natural environments among the rich genetic resources of Cappadocia (Nevşehir-Turkey) was determined
by artificial freezing tests in controlled conditions. Apricot cultivars of Hacıhaliloğlu, Kabaaşı, Hasanbey,
Aprikoz and Levent were used as control. Low temperatures were applied to flower buds, flowers and young
fruits at red calyx (–8 °C), balloon (–8 °C), full flowering (–4 °C), petal fall (–3 °C) and young fruit (–3 °C) stages
for 2 hours. The cooling rate was 2 °C h−1 with linear decline. Survival rates (%) of female organs in flowers and
seeds in young fruits were determined by visual assesments, and by electrical conductivity (μS.cm−1) measurements which is an indication of ion leakage from damaged tissues in genotypes. The results showed that
resistance of the wild apricots to low temperatures varied by developmental stage. Overall, most of the genotypes had higher survival rates than the standard apricot cultivars. However, genotypes # 24 and 45 exhibited
high viability rates (68.3% <) and low EC values (< 30.1%) at many stages of development in both years, and
were considered resistant to spring late frosts. Hierarchical Cluster Analysis with the genotypes’ survival rate (%)
and electrical conductivity (μS cm−1) data together without discrimination of growth stage placed these two
genotypes in the same group.
1. Introduction
Turkey has been dominating the world apricot production for a long
time. Apricots are grown on 123.800 ha land and current fresh fruit
production has reached to 730.000 tons supplying 18.8% of the world
production (3.9 million tons) (FAO, 2016). The most important problem encountered in the cultivation is the spring late frost damage, as
are all over the world. Temperatures drop below zero degree at sensitive developmental stage from the swelling of flower buds to the young
fruits can cause large crop losses, even entire crop in some years. Fruit
trees use similar strategies including avoidance and tolerance to cope
with frost stress. Obviously the most effective and reliable method
against late spring frosts in apricots, thus avoiding frost damage, is late
flowering. The timing of flowering is genetically controlled, and may
⁎
exhibit large variation among the genotypes. Much of the breeding
effort has concentrated on the use of delayed flowering as a means of
frost avoidance. Development of low temperature resistant cultivars in
the spring season is also effective and reliable method against late
spring frosts in apricots. The selection of genotypes having this character among the wild apricot genetic resources, where the variation is
high, is of great importance in breeding studies (Carter et al., 1999;
Guerriero et al., 2006; Nazemi et al., 2016). Anadolu (Turkey), which is
located in the center of secondary origin of apricot, has a large genetic
diversity in this respect (Layne et al., 1996; Ercisli, 2004;
Zhebentyayeva et al., 2012). Cappadocia region (Nevsehir, a province
famous for fairy chimneys) in central Anatolia has 3rd largest wild
apricot tree population with over 145.000 trees in Turkey (TUIK, 2017),
and this rich genetic resources are studied for the first time in terms of
Corresponding author.
E-mail address: verdogan@agri.ankara.edu.tr (V. Erdogan).
https://doi.org/10.1016/j.scienta.2018.10.038
Received 2 August 2018; Received in revised form 11 October 2018; Accepted 15 October 2018
0304-4238/ © 2018 Elsevier B.V. All rights reserved.
Scientia Horticulturae 246 (2019) 347–353
H. Dumanoglu et al.
the resistance to spring late frosts in this study. In this region, growers
have been able to grow wild apricot trees on soils with volcanic tuff, a
very soft and porous stone, underneath without irrigation since tuff
holds moisture inside and the roots utilize this moisture during the
growing season.
Apricot has been defined as a sensitive species to freezing temperatures when flower buds have fulfilled the endodormancy with the
beginning of active growth. Fower buds reaching to pre-flowering
stages are characterized by a fast dehardening against sub-zero temperatures and become particularly sensitive to spring frosts. Heat
temperatures support the reactivation of bud growth, and vascular
continuity is established between the floral primordium and the fruiting
shoot (Ashworth and Rowse, 1982; Rodrigo, 2000; Bartolini et al.,
2006a,b; Szalay et al., 2006; Kaya et al., 2018). Apricot genotypes can
react differently against frost stress in several ways such as ability to
deep supercool, maintaining water and dilute solution as metastable
liquids below their melting points (Ashworth, 1984) or activating
scavenging mechanisms enhancing the cells' resistance to frost such as
the formation of antioxidant enzymes (e.g. SOD) and an increase in
GSH/GSSG ratio (Doulis et al., 1993; Bartolini et al., 2006a).
Resistance to spring late frosts is assessed by a number of physiological events such as genotype, phenology, formation of ice nucleation,
moisture content and nutritional status. Although the frost damage is
largely dependent on the developmental stage of flower buds, the critical temperatures for freezing vary not only with the phenological
stage of flower buds but also with the genotype (Proebsting and Mills,
1978; Westwood, 2009; Rodrigo, 2000; Guerriero et al., 2006). Cold
sensitivity level of genotypes can be evaluated simply by observing
damage after severe frost events in orchard contitions. However, it is
important that sensitivity level of the genotypes is confirmed by
freezing tests in controlled environments. Laboratory tests allow the
measurement of freezing tolerance of tissues at different stages, and
evaluations can be performed visually by observing tissue browning
and/or other analytical procedures (Guerriero et al., 2006; Bartolini
et al., 2006a). Electrolyte leakage test, within the analytical procedures,
is a simple, rapid, reliable and effective indicator of frost resistance of
selected genotypes. Infact, electrolyte leakage measurement has long
been used to estimate cell membrane permeability in environmental
stress, growth and development, genotypic variation studies (Whitlow
et al., 1992; Imani et al., 2011; Perez-Harguindeguy et al., 2013; Lu
et al., 2017). As an injured cell is unable to maintain the chemical
composition of its contents it may, therefore, release electrolytes
through its membrane ofwhich selective permeability has been damaged when exposed to freezing temperaures. Loss of electrolytes can
be determined by washing excised tissues in water and measuring the
conductivity of the resultant solution. Conductivity of water extracts
are used as direct measurements of the extent of damage caused by
freezing (Levit, 1980; Murray et al., 1989). Similarly, level of integrity
of the cellular membranes of tissues can be estimated by Phenolic
Compound Leakage (PCL) test since PCL rate has been related to the
survival of bud and consequently to the bearing aptitude (Viti et al.,
2010).
The objective of this study was to apply artificial freezing tests in
controlled conditios to flower buds, flowers and young fruits of 36
promising wild apricot genotypes selected from Cappadocia region
since they were not affected by severe spring late frosts, and to evaluate
their resistance to frost by visual assesment of tissue viability and by
measurement of electrical conductivity (EC).
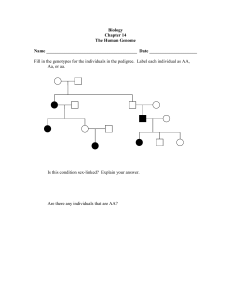
region (Nevşehir) in Anatolia was determined by freezing tests in
controlled conditions (Fig. 1). The Cappadocia region is a place where
late spring frosts occur intensively almost every year. At the end of
March in 2014, several late spring frosts took place successively at
between −7 °C and −13 °C, and caught the trees at swelled bud, flower
or young fruit stages resulting in a loss of 100% of the crop in the region
regardless of location or altitude. However, our field surveys covering
the area of about 2800 km2 at elevation ranging from 910 m and
1595 m showed that some trees did not get affected by the frosts seriously and were able to produce fruits unlike the many trees in their
surroundings. These trees were recorded and examined in detail following three years. The genotypes included in this study were the
genotypes selected in those studies. Their flowering dates as compared
to the others at the same location and at the same elevetion were given
in Table 1. Standard Turkish apricot cultivars of Hacıhaliloğlu, Kabaaşı,
Hasanbey, Aprikoz and Levent were included in the study as the control.
Branches carrying flower buds, flowers and young fruits were
collected from the trees, and immediately brought by night shipment to
the lab at Department of Horticulture, Faculty of Agriculture, Ankara
University, Ankara, Turkey.
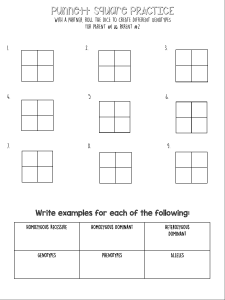
2.1. Freezing tets
In freezing tests, phenological stages and low temperatures reported
by Ballard et al. (1994) were followed that temperatures of −8, −8,
−4, −3 and −3 °C were applied to flowers at red calyx, first white
(balloon), full bloom, petal fall (in shuck) and to young (green) fruits,
respectively (Fig. 2).
Freezing tests were conducted in laboratory conditions. A horizontal
chest freezer was modified and controlled by a computer program
(Etha, KDD-23) to facilitae stable temperature decline. The system was
similar to that of Nesbit et al. (2002). The freezer was equipped with
two fans of 10 cm diameter on opposite false walls for ensuring
homogeneous temperature inside the chamber. A heating fan was
mounted next to the freezer, and pipe having halls laid in the chamber
was connected to this fan through an opening on the false wall. The
temperature was determined as average of by two RT/Pt100 thermometers mounted in the chamber, and recorded at every 5 s by a digital
data recorder (Elimko, E-PR-110). During a run, the compressor was left
on wheras the heating fan was activated to push warm air through the
pipe by the computer as often as necessary. This system produced a
linear decline with temperature fluctuating at most 0.3 °C about the
mean.
Before the tests, the branches were kept in a refrigerator (+4 °C) for
at least 2 h, then placed in the freezer. Chamber temperature was
lowered from 0 °C to test temperature where the branches were kept for
2 h, then returned to 0 °C. Cooling or heating rate was 2 °C h−1 (Öztürk
et al., 2000; Barranco et al., 2005; Viti et al., 2010). The branches were
then removed and allowed to thaw at 4 °C in water for 12 h for visual
frost damage assesments, or for 4 h for electrical conductivity measurements.
2.2. Visual frost damage assesments
After thawing the branches were incubated at room temperatures
for 24 h. Frost injury was visually rated. The flower buds, flowers and
young fruits were bisected longitudinally, and pistils and seeds were
observed under a stereomicroscope. Samples appeared bright and green
was considered alive and that appearing black/brown was considered
dead (Rekika et al., 2004; Viti et al., 2010). Evaluations were made on
50 samples, and the rate of viability was expressed as a percentage.
2. Materials and methods
In this study, frost resistance of 36 wild apricots from Cappadocia
348
Scientia Horticulturae 246 (2019) 347–353
H. Dumanoglu et al.
Fig. 1. Cappadocia region (Nevşehir) in Central Anatolia, Turkey, where 36 local wild apricot genotypes were selected.
Genotypes with viability rates of 50%≤ in two years were considered
highly frost resistant.
Table 1
Flowering dates of selected wild apricot genotypes used in the study as compared to the others at the same location and at the same elevation.
Genotype no
3
4
6
7
13
14
15
16
17
18
19
21
22
24
26
27
28a
29
31
32
33
34
35
38
39
41
42
43
45
46
47
48
49
50
53
54
a
2015
2016
2.3. Electrical conductivity (EC) measurements
2017
Others
Selected
Others
Selected
Others
Selected
06/04
06/04
18/03
06/04
30/03
30/03
30/03
30/03
06/04
07/04
23/03
23/03
23/03
14/04
14/04
14/04
14/04
14/04
14/04
14/04
14/04
14/04
14/04
16/03
18/03
06/04
06/04
06/04
14/04
14/04
14/04
14/04
14/04
14/04
14/04
14/04
21/04
21/04
31/03
20/04
07/04
07/04
30/03
07/04
21/04
30/03
07/04
31/03
15/04
20/04
20/04
20/04
04/05
27/04
20/04
20/04
20/04
27/04
20/04
23/03
31/03
06/04
14/04
14/04
–
–
–
–
–
–
–
–
17/03
17/03
07/03
17/03
07/03
14/03
14/03
14/03
17/03
25/03
11/03
07/03
21/03
25/03
25/03
25/03
25/03
25/03
25/03
25/03
25/03
25/03
25/03
29/02
07/03
14/03
17/03
17/03
25/03
25/03
25/03
25/03
25/03
25/03
25/03
25/03
31/03
28/03
11/03
01/04
25/03
28/03
23/03
28/03
21/03
07/03
21/03
11/03
24/03
04/04
04/04
04/04
08/04
04/04
04/04
04/04
04/04
04/04
04/04
07/03
11/03
21/03
31/03
24/03
04/04
04/04
31/03
01/04
04/04
04/04
28/03
03/04
09/04
09/04
22/03
09/04
03/04
03/04
03/04
03/04
09/04
20/04
03/04
03/04
03/04
17/04
17/04
17/04
17/04
17/04
17/04
17/04
17/04
17/04
17/04
21/03
22/03
09/04
09/04
09/04
17/04
17/04
–
17/04
17/04
17/04
17/04
17/04
17/04
17/04
09/04
17/04
13/04
18/04
10/04
–
–
03/04
–
03/04
–
24/04
17/04
17/04
30/04
24/04
24/04
24/04
24/04
24/04
24/04
21/03
03/04
17/04
17/04
17/04
17/04
18/04
–
24/04
18/04
18/04
17/04
23/04
In addition to visual evaluations, frost injury was determined as
electrolyte leakage by measuring electrical conductivity (μS.cm−1) of
the leachate from the damaged tissues. Electrolyte leakage is generally
considered as an indirect measure of plant cell membrane damage, and
plants with low relative EC values were considered more resistant to
frost (Barranco et al., 2005; Imani et al., 2011). After thawing, 0.5 g
samples were washed in deionized water. The samples in 15 ml deionized water in erlenmeyer flasks were shaken at 120 rpm in light conditions at room temperature for 24 h. Then, the initial electrical conductivity (initial EC) was measured. The samples were then autoclaved
(1 h, 120 °C, 1 atm) to kill the tissues completely. After 2 h shaking at
200 rpm in light conditions electrical conductivity was measured again
(autoclave EC) to obtain a reference value for total ions. Relative EC at
each test temperature was calculated as;
Relative EC = (initial EC/autoclave EC) × 100
Genotypes with relative EC values of ≤ 50% in two years were
considered highly frost resistant.
2.4. Statistical analysis
The results of the survival rate and electrical conductivity measurements at five different phenological stages in 41 apricot genotypes
including the controls were taken together, and the genotypes showing
similar performance in all stages were classified using the Ward's
Method and Squared Euclidean distance in the Hierarchical Cluster
Analysis method. Calculations were performed with IBM SPSS Statistics
Version 20 software package.
The latest wild apricot genotype to flower in the study.
349
Scientia Horticulturae 246 (2019) 347–353
H. Dumanoglu et al.
Fig. 2. Phenological stages at which freezing tests were applied in wild apricot genotypes. From left to right; red calyx, first white (balloon), full bloom, petal fall (in
shuck) and young (green) fruit (Ballard et al., 1994).
3. Results and discussion
Table 2
The average viability rates of female organs in flowers or seeds in young fruits
at different phenological stages after the freezing tests in wild apricot genotypes
selected from Cappadocia region (Turkey), and the cultivars.
The survival rate of flower buds, flowers and young fruits is an
important indicator for whether or not the tree will show resistance
when hit by the spring late frosts in nature. Changes in the hardening
and dehardening period may occur due to weather, abiotic and biotic
factors in the previous season, affecting the sugar accumulation in wood
and its frost resistance. Thus we repeated the freezing tests to get reliable data. A genotype that is caught by the frost several times successively in the spring in the red calyx, flower or young fruit stage, may
not get damaged due to tissues’ resistance level within acceptable
limits. In our study, visual ratings of female organs and seeds for frost
damage under stereomicroscope revealed that the resistance of the wild
apricots selected from Cappadocia region to low temperatures varied by
the phenological stage of the sample. Viability (%) of female organs and
seeds of the genotypes and the cultivars after the freezing tests are given
in Table 2.
Flower buds at red clayx stage were subjected to freezing temperature at −8 °C for 2 h in the experiment. At this stage, the average
viability rate of female organs ranged from 11.8% (# 28) to 89.0% (#
3) among the wild apricot genotypes and from 25.7% (Aprikoz) to
52.0% (Levent) among the cultivars. However, the genotypes # 3, 13,
19, 24, 45, 46, 48, 49, 53 and 54 had viability rates of 50% ≤ in both of
the years. Especially, genotypes # 3, 13, 45, 48, 49 and 53 showed over
80% survival rate in average (Table 2). The rest of the genotypes and
the apricot cultivars were not able to show survival rate of 50% in both
of the years. Özkarakaş (2002), reported that Septic was the most resistant cultivar at red clayx stage with survival rate of 93.3-94.6% at
−3°C and 56.9-66.3% at −5°C among the apricot cultivars of Alyanak,
Çiğli, Şekerpare and Yahudi. However, we applied lower test temperature (–8 °C) than the researcher in freezing tests and found over
85% survival rate on average in the genotypes # 3, 13, 45, 48 and 49.
Although Öztürk et al. (2006) recorded 100% damage at −7 °C at the
red clayx stage in Hasanbey, Hacı Haliloğlu, Çataloğlu, Soğancı and
Kabaaşı cultivars we found 66.8%, 71.2% and 68.4% injury level at this
stage in Hasanbey, Hacıhaliloğlu and Kabaaşı cultivars, respectively.
Freezing temperature at −8 °C was applied to the flowers at the
balloon (first white) stage for 2 h. The average viability rate of female
organs ranged from 0.0% (# 20 and 49) to 80.0% (# 45) in wild apricot
genotypes, and from 9.6% (Hasanbey) to 35.3% (Aprikoz) among the
apricot cultivars (Table 2). We recorded 50% ≤ viability in genotypes
# 4, 13, 24, 42 and 45 in both of the years of the experiment, while
genotype # 45 had the highest survival rate (80%). The rest of the
genotypes and the apricot cultivars had lower viability (< 50%) in both
years. Gunes (2006) reported that flowers of Hacıhaliloğlu and Kabaaşı
cultivars at balloon stage tested at −4 °C for 1–3 h had 81.1% and
95.2% survival, respectively. These values are well above than what we
obtained in Hacıhaliloğlu (14.0%) and Kabaaşı (14.2%) cultivars. Apparently, the reason for this extreme difference is the test temperature
which was two times lower (at −8 °C for 2 h) in our experiment, representing severe frost condition, than the temperature applied by the
researcher.
The flowers at the full bloom stage were treated with low temperature of −4 °C for 2 h in the tests. The average viability rate in
Genotype No
3
4
6
7
13
14
15
16
17
18
19
21
22
24
26
27
28
29
31
32
33
34
35
38
39
41
42
43
45
46
47
48
49
50
53
54
Aprikoz
Kabaaşı
Hasanbey
Hacıhaliloğlu
Levent
a
Survival rate (%)
Red calyx
(-8 °C)
First white
(-8 °C)
Full bloom
(-4 °C)
Petal fall
(-3 °C)
Young
fruit
(-3 °C)
89.0a
42.1
69.8
36.8
85.0a
56.8
55.6
47.9
44.0
62.0
65.9a
67.6
27.7
68.3a
34.0
33.5
11.8
48.4
36.7
22.3
23.3
35.1
39.0
31.0
55.0
41.9
52.8
33.9
84.9a
62.6a
24.0
86.0a
85.0a
41.0
81.0a
69.0a
25.7
37.6
43.5
35.5
52.0
67.9
66.2a
32.8
12.5
59.0a
60.4
17.0
33.8
5.0
46.8
60.0
21.6
25.0
73.4a
22.3
21.2
12.0
47.3
21.2
10.0
17.2
28.3
33.1
14.7
21.3
40.3
63.8a
9.5
80.0a
9.2
54
20.9
0.0
20.0
62.0
45.0
35.3
14.2
9.6
14.0
34.6
67.8a
82.0a
66.0
90.7a
49.5
42.4
92.8a
42.1
64.5
25.9
54.9
57.2
40.5
81.7a
93.8a
69.6a
63.3
92.0a
70.6
71.6a
79.0a
61.0
69.0a
33.9
25.7
58.5
39.4
52.3
100a
92.0
53.3
43.3
64.2
84.0a
61.0
94.0a
30.7
21.9
6.0
14.8
51.8
49.3
56.2
97.8a
48.0
67.4
38.7
75.0a
40.4
25.7
57.5
56.1a
81.2a
56.7
72.9a
51.5
92.5a
42.0
64.9
42.9
32.4
47.0
24.0
55.6
22.7
38.4
49.0
64.0a
85.8a
75.9a
53.0
60.3
74.3a
67.7
37.5
24.7
3.0
25.0
9.0
11.8
16.8
33.6
50.0
75.7a
53.8
56.2
68.2
48.9
97.6a
90.0a
60.0
47.7
59.5
88.3
48.2
57.4
63.5
73.3
27.9
44.0
59.6
45.3
20.0
60.9
49.3
16.2
5.5
75.0a
55.2
87.5a
41.4
32.5
71.8
41.6
25.6
41.0
70.4a
60.0
30.0
20.0
30.0
42.9
55.2
Genotypes with viability rates of 50% or more in two years were considered
female organs ranged from 25.7% (# 39) to 100% (# 45) among the
wild apricots (Table 2). Genotypes # 3, 4, 7, 15, 24, 26, 27, 29, 32, 33,
35, 45, 50 and 54 consistently exhibited 50% ≤ viability in both of the
years. Especially, genotypes # 4, 7, 15, 24, 26, 29, 45, 50 and 54
showed over 80% viability on average. Among the cultivars, viability of
female organs were found between 6.0% (Hasanbey) and 51.8% (Levent) on average, however none of the cultivars showed 50% or more
survival rate consistantly. Gunes (2006) reported much higher survival
350
Scientia Horticulturae 246 (2019) 347–353
H. Dumanoglu et al.
rates of 78.3% in Hacıhaliloğlu and 89.1% in Kabaaşı cultivars tested at
full bloom at −4 °C for 1–3 h in a single year study than what we found
on these cultivars (14.8% and 21.9%, respectively) (Table 2).
Özkarakaş (2002), found the highest viability of 95.4% and 89.9% in
Septik cultivar at full bloom after low temperature treatments at −3 °C
for 2 h and 3 h, respectively. In other cultivars, viability rates were
95.3% in Şekerpare, 85.2% in Çiğli, 49.6% in Yahudi and 42.5% in
Alyanak at −3 °C for 2 h. On the other hand, tests at −5 °C for 2 h
resulted in 80.0% damage in Şekerpare while the others had higher
level of damage. Our results indicated that the wild apricot genotypes
had better performance interms of viability rate while the standard
cultivars had serious damage in contratry to reports of Gunes (2006)
and Özkarakaş (2002). However, injury levels of the cultivars in our
study were lower than the ones reported by Öztürk et al. (2006) who
observed 100% of flower death at full bloom at −4 °C in Hasanbey,
Hacıhaliloğu, Çataloğlu, Soğancı and Kabaaşı cultivars.
We applied freezing tests (–3 °C for 2 h) to flowers at petal fall, and
the genotypes # 6, 15, 19, 21, 24, 27, 42, 43, 45 and 48 had
50% ≤ viability consistently in both of the years. Higher viability rates
were found in genotypes # 6 (97.8%), 21 (81.2%), 27 (92.5%) and 43
(85.8%) than the others (81.2%). The standard cultivars showed serious
female organ injury at this stage, and the survival rate varied between
9.0% (Kabaaşı) and 33.6% (Levent) on average (Table 2). Özkarakaş
(2002) reported that the cultivar Septic showed higher tolerance after
treatment of cold temperature (–3 °C) than Alyanak, Yahudi, Çiğli and
Şekerpare cultivars. On the other hand, Öztürk et al. (2006), found
100% injury at temperature of −2 °C in Hasanbey, Hacıhaliloğu, Çataloğlu, Soğancı and Kabaaşı cultivars. These results are in line with
ours.
Young fruits were tested at −3 °C for 2 h, and the genotypes # 4, 15,
16, 41, 43 and 53 were recorded with 50% ≤ viability in both of the
years. Among these genotypes, # 15, 16 and 43 had 87.5%≤ viability
on average (Table 2). On the other hand, the standard cultivars had low
levels of viability ranging from 20.0% (Kabaaşı) to 55.2% (Levent) on
average.
Although several genotypes were noted for higher survival rates
after freezing tests at different stages, the genoypes # 15, 24 and 45
gave better results at more than one stage. Among these, genotype # 15
survived at test temperatures in full bloom, petal fall and young fruit
stages in both years of the experiment, while genotypes # 24 and 45
performed better in red calyx, first white, full bloom and petal fall
stages. Survival rate of female organs in these genotpes were found
quite high (68.3% <) at all stages, except young fruit stage (Table 2).
In general, most of the wild apricot genotypes selected from Cappadocia
region had lower levels of frost damage than the standard cultivars of
which flower buds, flowers or young fruits were not able to show viability 50% and over in two years.
In this study, freeze injury was also determined by measuring
electrical conductivity (μS.cm−1) which is an indicator of electrolyte
leakage from the tissues after low temperature treatments. The relative
EC values (%) of flowers and young fruits after the freezing tests in wild
apricot genotypes selected from Cappadocia region, and the standard
cultivars (control) are given in Table 3. At red calyx stage, genotypes #
3, 6, 7, 17, 18, 21, 24, 29, 38, 43, 45, 46, 48, 49, 50, 53 and 54 had EC
values of ≤50% in both years. Relative EC values were quite low
especially in genotypes # 54 (9.9%), 53 (10.7%), 21 (16.1%) and 7
(16.9%) on average. Apricot cultivars except Aprikoz had relative EC
values below 50% (Table 3), and the lowest value (25.8%) was recorded in Levent cultivar. Viti et al. (2010) applied freezing tests at
−4 °C for 2 h at the bud break stage when pinkish tips of sepals begun
to appear in the apricot collection plot located in Tyrrhenian coastal
region of Italy and determined the frost sensitivity of the genotypes by
quantifying the phenolic substances leaked. They found that leakage
percentage varied among the genotypes from 11% (tolerant) to 83%
(sensitive).
In balloon stage, relative EC values of genotypes # 4, 21, 24, 26, 27,
Table 3
The average relative electrical conductivity values of female organs in flowers
or seeds in young fruits at different phenological stages after the freezing tests
in wild apricot genotypes selected from Cappadocia region (Turkey), and the
cultivars.
Genotype No
3
4
6
7
13
14
15
16
17
18
19
21
22
24
26
27
28
29
31
32
33
34
35
38
39
41
42
43
45
46
47
48
49
50
53
54
Aprikoz
Kabaaşı
Hasanbey
Hacıhaliloğlu
Levent
a
Electrical conductivity (%)
Red calyx
(-8 °C)
First white
(-8 °C)
Full bloom
(-4 °C)
Petal fall
(-3 °C)
Young
fruit
(-3 °C)
29.7a
39.2
18.0a
16.9a
42.1
36.2
33.9
13.8
22.3a
21.7*
11.5
16.1a
22.1
22.0a
46.7
40.5
41.1
32.5a
36.9
51.4
39.9
51.3
43.4
21.2a
21.1
37.9
48.0
30.8a
20.0a
19.3a
8.1
20.1a
13.9a
23.9a
10.7a
9.9a
43.8
31.6a
33.2a
28.8a
25.8a
55.2
34.6a
55.0
70.5
49.0
55.4
44.4
43.6
36.7
53.4
35.0
26.9a
27.3
30.1a
21.7a
32.6a
63.5
32.1a
38.5a
38.8a
40.9
52.5
50.9
29.0a
23.9a
36.2a
56.1
33.7a
20.7a
35.5a
28.7
27.6a
19.8a
38.6a
33.1a
44.3a
39.1
47.8
38.6a
36.1a
38.3a
37.5a
53.8
19.4a
33.2a
45.1
53.8
43.8
39.4
38.5
50.2
29.8
26.3a
28.2
22.4
32.5a
39.8
41.5
24.9a
36.9
41.1
43.9a
49.9
35.2
49.2
41.5
41.3
54.0
61.4
28.8a
36.4
47.1
52.1
50.3
27.6a
28.9a
29.1a
45.4
49.7
41.5a
55.6
28.3a
68.0
50.6
39.2
72.0
62.3
43.4
56.2
79.0
85.1
82.2
49.9
73.3
52.0
66.2
52.3
56.8
55.1
75.8
59.0
72.7
51.8
74.1
41.9a
71.4
43.5a
39.4
47.3
52.2
65.6
52.3
57.8
52.7
73.9
55.3
67.7
84.6
56.4
65.7
63.4
70.1
36.4a
55.6
40.4
41.3
61.5
26.0a
53.7
23.7a
43.1
52.6
61.9
25.4
28.9
47.8
4.0a
8.9
40.4
69.2
38.2
51.9
38.3
33.0a
43.8
56.2
74.8
94.2
13.3a
50.3
44.6
67.2
70.8
46.2
29.0
70.8
54.0
42.7
13.8
57.5
83.3
66.7
67.3
26.6
Genotypes with EC values of 50% or lower in two years were considered as
29, 31, 32, 38, 39, 41, 43, 45, 46, 48, 49, 50, 53 and 54 were recorded
below 50% in both of the years. Among these, genotypes # 26 (21.7%), 39
(23.9%), 45 (20.7%) and 49 (19.8%) had quite low EC values. Similarly,
EC of Hasanbey, Hacıhaliloğlu and Levent cultivars were also below 50%
(Table 3). In full bloom, genotypes # 3, 6, 7, 21, 26, 29, 33, 45, 50, 53 and
54 gave EC below 50% in both of the years (Table 3). In some of these
genotypes (# 3, 7, 26, 29, 33, 45, 50 and 54) flower survival rates were
also higher (Table 2). Although, Hasanbey and Levent cultivars showed EC
below 50% in both of the years, other cultivars did not have reliable results. At this stage, none of the cultivars showed a flower viability over
50% after the freezing tests in all years (Table 2). In petal fall, relative EC
was found below 50% in only genotypes # 35 and 39 in both of the years
(Table 3). None of the standard cultivars had EC below 50% except Levent
(36.4%). In young fruit stage, average ion leakage was low in genotypes #
13 (26.0%), 15 (23.7%), 24 (4.0%), 33 (33.0%) and 41 (13.3%) in two
years (Table 3). It is also noteworthy that genotypes # 15 and 41 had also
high seed viability rates (Table 2). At this stage, the cultivars were found
sensitive to frosts which was indicated by high EC values and lower survial
rates (Table 3).
351
Scientia Horticulturae 246 (2019) 347–353
H. Dumanoglu et al.
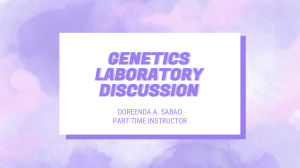
Fig. 3. Dendogram of wild apricot genotypes and standard cultivars (control) after analysis of survival rate (%) and electrical conductivity (μS cm−1) data together in
all phenological stages using the Ward's Method and Squared Euclidean distance in the Hierarchical Cluster Analysis method (standard cultivars; 81: Aprikoz, 82:
Kabaaşı, 83: Hasanbey, 84: Hacıhaliloğlu, 85: Levent).
We found that, relative EC values highlighted some genotypes at
some stages, as in viability rates. However genotypes # 21, 24, 29, 45,
50, 53 and 54 had low relative EC values in more stages than the others
in two years (Table 3). Especially genotypes # 24 and 45 deserve more
attention. The standard cultivars were used as control in the experiment. Among them, Hasanbey and Levent were recoreded with low
relative EC values in red calyx, balloon, full bloom, and petal fall (Levent only) in two years, while viability rates in these cultivars did not
exceed 50% in both of the years (Table 2). Evaluation of cell integrity,
enabled us to determine the frost tolerance and the degree of injuries of
flower buds and young fruits. Viti et al. (2010), reported that the artificial frost treatment associated with cell integrity test ascertains the
performance of genotypes against frost tolerance. This method could be
used for accelerating breeding programs with the objective of developing frost hardy apricot cultivars.
The clusters and groups presented in Fig. 3 and Table 4 were obtained after analysis of the genotypes’ survival rate (%) and electrical
conductivity (μS.cm−1) data together without discrimination of growth
stage with Hierarchical Cluster Analysis. The genotypes most sensitive
to freezing were placed in Group 5 (Table 4) that most of them were
standard cultivars which were clustered together in the dendogram as
well (Fig. 3). On the other hand, the genotypes placed in the first group
were more resistant to frost treatments. Infact, genotypes # 24 and 45
were placed in this group as they were identified frost-resistant genotypes which showed better results in more phenological stages after
frost treatments (Tables 2–4). As expected, they were placed in the
same cluster in the dendogram.
4. Conclusion
Late flowering is a way of frost avoidance that these genotypes are
less likely to experience frost. However, late flowering genotypes might
be frost sensitive. No relationship was found between late flowering and
frost resistance in this study. For example, genotypes # 28 was the
latest to flower but viability rates and EC values were not superior. Wild
apricot genotypes # 24 and 45 were found promising resistant genotypes to spring late frosts as they expressed higher survival rates in
female organs, and lower ion leakage from the tissues in most of the
stages of red calyx, balloon, full bloom, petal fall and/or young fruit
after freezing tests in controlled conditions. These genotypes affected
slightly and produced resasonable amounts of crop after successive
severe frosts (between −7 and −13 °C) occurred in Cappadocia region
at the end of March in 2014 where more than hundred thousands of
wild apricot trees damaged and lost their entire crop in that year. In
addition, the genotype # 24 flowered at least a week later than that of
the genotypes in the surrounding area during 2015–2017 while no late
Table 4
Classification of wild apricot genotypes and standard cultivars (control)
based on survival rate (%) and electrical conductivity (μS cm−1) data together in all phenological stages after analysis using the Ward's Method
and Squared Euclidean distance in the Hierarchical Cluster Analysis
method. Group numbers from 1 to 5 represents resistance level from more
resistant to more sensitive. Standard cultivars are Aprikoz (#81), Kabaaşı
(#82), Hasanbey (#83), Hacıhaliloğlu (#84) and Levent (#85).
Group
Genotype code
1
2
3
4
5
3, 4, 13, 14, 18, 19, 24, 29, 42, 45, 53, 54
6, 46, 48, 49
7, 17, 28, 31, 32, 33, 34, 35, 50
15, 16, 21, 22, 26, 27, 41, 43, 47, 85
38, 39, 81, 82, 83, 84
352
Scientia Horticulturae 246 (2019) 347–353
H. Dumanoglu et al.
flowering was observed in genotype # 45. Scionwoods of selected
genotypes were grafted and planted in a reaseach plot of Department of
Horticulture, Faculty of Agriculture at Ankara University in Ankara.
Further investigations will be done to compare them at the same location.
241, 322–328.
Layne, R.E.C., Bailey, C.H., Hough, L.F., 1996. Apricots. In: Janick, J., Moore, J.N. (Eds.),
Fruit Breeding, Vol 1: Tree and Tropical Fruits. Jhon Viley & Sons, Inc., New York, pp.
79–111.
Levit, U., 1980. Responses of plants to environmental stresses. In: In: Kozlowski, T.T.
(Ed.), Chilling, Freezing and High Temperature Stresses, vol. 1. Academic Press, New
York, pp. 256–258.
Lu, Y., Hu, Y., Li, P., 2017. Consistency of electrical and physiological properties of tea
leaves on indicating critical cold temperature. Biosyst. Eng. 159, 89–96.
Murray, M.B., Cape, J.N., Fowler, D., 1989. Quantification of frost damage in plant tissues
by rates of electrolyte leakage. New Phytol. 113, 307–311.
Nazemi, Z., Zeinolabedini, M., Hallajian, M.T., Bouzari, N., Majidian, P., Ebrahimi, M.A.,
2016. Assessment of Iranian apricot cultivars resistant, susceptible and mutant to late
spring frost. J. Plant Mol. Breed. 4 (2), 9–16.
Nesbit, M.L., Ebel, R.C., Findley, D., Wilkins, B., Woods, F., Himelrick, D., 2002. Assays to
assess freeze injury of Satsuma mandarin. HortScience 37 (6), 871–877.
Özkarakaş, İ., 2002. Determination of resistance to spring frost of some commercial
apricot (P. armeniaca L.) cultivars grown in Aegean region. Anadolu 12 (2), 35–48.
Öztürk, K., Küden, A., Güloğlu, U., Ölmez, H.A., Çelik, B., Çolak, S., 2000. Malatya’da
yetiştirilen bazı kayısı çeşitlerinin kış soğukları ve ilkbahar geç donlarına dayanımları
üzerine araştırmalar. Sonuç Raporu. Malatya Meyvecilik Araştırma Enstitüsü
Müdürlüğü, Malatya.
Öztürk, K., Ölmez, H.A., Guloglu, U., Kuden, A., 2006. Evaluation of the resistance of
some apricot varieties growing in Malatya to winter hardiness and late spring frost.
Acta Hortic. 701, 247–252.
Perez-Harguindeguy, N., Diaz, S., Garnier, E., Lavorel, S., Poorter, H., 2013. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot.
61, 167–234.
Proebsting, E.L., Mills, H.H., 1978. Low temperature resistance of developing flower buds
of six deciduous fruit species. J. Am. Soc. Hortic. Sci. 103, 192–198.
Rekika, D., Cousineau, J., Levasseur, A., Richer, C., Fisher, H., Khanizadeh, S., 2004. The
use of a bud freezing technique to determine the hardiness of 20 grape genotypes.
Acta Hortic. 640, 207–212.
Rodrigo, J., 2000. Spring frosts in deciduous fruit trees − morphological damage and
flower hardiness. Sci. Hortic. 85, 155–173.
Szalay, L., Pappy, J., Pedryc, A., Szabo, Z., 2006. Influence of the changing climate on
flower bud development of apricot varieties. Acta Hortic. 717, 75–78.
TUIK, 2017. Number of Wild Apricot Trees in Nevşehir. Turkish Statistical Institute.
https://biruni.tuik.gov.tr/medas/?kn=92&locale=tr.
Viti, R., Bartolini, S., Andreini, L., 2010. Flower bud frost tolerance of several Italian
apricot genotypes. Eur. J. Hortic. Sci. 75 (5), 185–192.
Westwood, M.N., 2009. Temperate-Zone Pomology: Physiology and Culture, 3rd ed.
Timber Press, Portland.
Whitlow, T.H., Bassuk, N.L., Ranney, T.G., Reichert, D.L., 1992. An improved method for
using electrolyte leakage to assess membrane competence in plant tissues. Plant
Physiol. 98, 198–205.
Zhebentyayeva, T., Ledbetter, C., Burgos, L., Llacer, G., 2012. Apricot. In: Badenes, M.L.,
Byrne, D.H. (Eds.), Fruit Breeding. Springer, New York, pp. 415–458.
Acknowledgements
This research was supported by TUBITAK (The Scientific and
Technological Research Council of Turkey), project no 114O279.
Assistance of Nevşehir Directorate of Provincial Agriculture and
Forestry in field studies in Cappadocia region were greatly appreciated.
References
Ashworth, E.N., Rowse, J.D., 1982. Vascular development in dormant Prunus flower buds
and its relationship to supercooling. HortScience 17, 790–791.
Ashworth, E.N., 1984. Xylem development in Prunus flower buds and the relationship to
deep supercooling. Plant Physiol. 74, 862–865.
Ballard, J.K., Proebsting, E.L., Tukey, R.B., 1994. Critical Temperatures for Blossom Buds:
Apricots. WSU Research and Extension Center (Reprint, Pub no: EB1240).
Barranco, D., Natividad, R., Campo, M.G., 2005. Frost tolerance of eight olive cultivars.
HortScience 40 (3), 558–560.
Bartolini, S., Zanol, G., Viti, R., 2006a. The cold hardiness of flower buds in two apricot
cultivars. Acta Hortic. 701, 141–146.
Bartolini, S., Viti, R., Guerriero, R., 2006b. Xylem differentiation and microsporogenesis
during dormancy of apricot flower bud. Eur. J. Hortic. Sci. 71, 84–90.
Carter, J., Brennan, R., Wisniewski, M., 1999. Low-temperature tolerance of blackcurrant
flowers. HortScience 34 (5), 855–859.
Doulis, A.G., Hauslanden, B., Mondy, B., Alscher, R.G., Chevone, B.I., Hess, J.L., Weiser,
R.L., 1993. Antioxidant response and winter hardiness in red spruce (Picea rubens
Sarg.). New Phytol. 123, 365–374.
Ercisli, S., 2004. A short review of the fruit germplasm resources of Turkey. Genet. Res.
Crop Evol. 51, 419–435.
FAO, 2016. Apricot Production. http://www.fao.org/faostat.
Guerriero, R., Viti, R., Bartolini, S., Iacona, C., 2006. Parents for spring frost tolerance in
apricot. Acta Hortic. 717, 153–156.
Gunes, N.T., 2006. Frost hardiness of some Turkish apricot cultivars during the bloom
period. HortScience 41 (2), 310–312.
Imani, A., Barzega, K., Piripireivatlou, S., 2011. Relationship between frost injury and ion
leakage as an indicator of cold hardiness in 60 almond selections. Int. J. Nuts Relat.
Sci. 2, 22–26.
Kaya, O., Kose, C., Gecim, T., 2018. An exothermic process involved in the late spring
frost injury to flower buds of some apricot cultivars (Prunus armenica L.). Sci. Hortic.
353