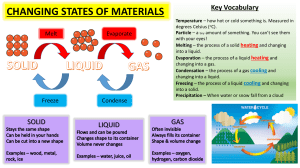
Hot Stamping Process Heating Phase The process begins with the heating of the blank up to the austenitization temperature. This temperature has a great influence on the part properties, the processing time and the cost-efficiency of hot stamping. Heating of the blank may be isothermal, non-isothermal or both. Li et al. [1] have investigated and characterized the effect of heating rate and temperature on the progress of austenite formation under both non-isothermal and isothermal conditions. The fraction and distribution of martensite in the formed part is determined by the extent of austenitization. Hence, the formation of austenite during the heating process is of primary importance in determining the final properties of a given part. Compared with the number of investigations on austenite decomposition during cooling, studies on austenite formation have been few. This is because the observation of the progress of austenite formation is difficult due to the difficulty in retaining the austenite at room temperature for inspection and characterization [2,3]. The material used is 22MnB5 (Manganese-Boron) steel with the chemical composition and initial microstructure information. Starting from a given initial microstructure, the heating rate and soaking temperature are two critical factors which affect the kinetics of austenitization. When a material undergoes a phase transformation, the lattice structure changes, which is in principle accompanied by a change in specific volume. The formation of austenite involves the lattice change of iron from a body-centered-cubic (BCC) structure to a face-centered-cubic (FCC) structure, which results in a change in density, hence volume. The hot stamping process begins with the heating of the blank op to the austenitization temperature. For the determination of the process window regarding a homogeneous austenitization of the blanks during a hot stamping as a pre-condition for fully martensitic transformation, lechler and Merklein [4] performed annealing tests (investigations) considering austenitization temperature and time. Starting from a given initial microstructure, the heating rate and soaking temperature are the two critical factors which affect the kinetics of austenitization [1]. The investigations of Lechler [5] have shown that the heating procedure of the blank has a great influence on the process time, the end mechanical properties of part, and the cost-efficiency of hot stamping. Therefore, a homogeneous blank temperature and a short heating time are the main demands on the heating system. There are two stages of heating phase: 1. Stage 1 - Continuous Heating: This stage involves heating to raise the temperature and phase transformation. This stage has two parts. a. First: It involves the heating which causes linear expansion in the material (Boron Steel in this case) with initial phase mixture. b. Second: Here, the expansion curve/heating curve deviates from linearity at 1007K (Ac1). This is because formation of austenite takes place. It is due to the competition between the volumetric change induced by phase transformation and thermal expansion. 2. Stage 2 - Soaking: This stage also provides heating at constant temperature. The purpose is to distribute the temperature uniformly throughout the material. At this stage, contraction is caused by the phase transformation. The austenite transformation rate in the isothermal soaking period decreases with the soaking time. Soaking time is usually 10-15 minutes. After soaking time, the final microstructure could be a mixture of austenite and ferrite. The phase transformation in general is controlled by two classes of factors: (1) Initial microstructure, including phase composition, chemical composition, grain size and the presence of nonmetallic inclusions; that is, the intrinsic properties of an alloy. (2) External conditions, including heating rate and temperature. Li et al. [1] performed two groups of experiments to investigate the effects of thermal conditions on austenite formation. The results are listed in table 1. In the first group of tests/experiments, soaking temperature was kept constant while heating rate and soaking time were different for each experiment in the first group. Results of the first group are discussed below: Effects of Heating Rate on Non-Isothermal Austenite Formation: It is apparent that less time is required at a higher heating rate to achieve the same volume fraction of austenite than at a lower heating rate. This is because higher heating rate stimulates a higher nucleation rate, which allows more nuclei for austenite formation to be generated even within a shorter period of time, thus enabling a higher overall growth rate of austenite [6]. Another effect of heating rate is that the temperature to attain a particular amount of austenite increases with increasing heating rate. This is mainly because a higher heating rate means less soaking time is available for diffusional transformation with a given growth geometry and the transformation would be shifted to higher temperatures [7,8]. The quantification of the heating rate effects on austenitization under continuous heating conditions is complex, since heating rate is coupled with both temperature and time. Nucleation and grain growth are the two primary mechanisms operating in the phase transformation process. Effects of Heating Rate on Isothermal Austenite Formation: The isothermal transformation takes place at a constant soaking temperature at which maximum austenite formation is possible. Results of experiments from [1] reveal that, at the same stage of the transformation under the same isothermal condition, less time is required for higher heating rates. It can be concluded that the thermodynamic effect of heating rate proceeds from the heating step and continues through the subsequent isothermal soaking step. This is because the transformation rate at any time depends on both the instantaneous growth rate of new phase grains and the existing quantity of grains [9]. Since more nuclei are generated during continuous heating at a higher rate, during the subsequent isothermal soaking at a given temperature, a larger amount of pre-existing grains enables a higher overall growth rate of new phase, i.e. a higher austenite formation rate. In the second group of tests/experiments, heating rate and soaking time were constant while socking temperature was different for each experiment in the group. Results of the second group are discussed below: Saturated Volume Fraction (ƒs): Heating rate affects the austenite transformation rate, but it doesn’t affect the maximum achievable value of austenite fraction at a particular soaking temperature, i.e. ƒs is only a function of temperature. Effects of Transformation on Isothermal Austenite Formation: Results reveal that less transformation time is required at a higher soaking temperature. Having the d=same heating rate, the same amount of nuclei should be generated in the tests. Then the transformation rate is controlled by the growth rate of the austenite phase. Thus, it is determined by only the soaking temperature under isothermal conditions. Higher soaking temperature enables faster progress towards equilibrium. The characterization of the dynamics of the austenite formation process is essential for the thermal condition design, e.g. heating rate and soaking temperature, to enhance productivity and reduce energy consumption in hot stamping process. Table 1. Experiments and their Results Test Group Test Group 1: Same Soaking Temperature (1173K) Test Group 2: Same Heating Rate (5 K/s) Thermal Conditions Heating Rate (K/s) Soaking Time (minutes) 1 10 5 15 25 15 Soaking Temperature Soaking Time (minutes) 1023 15 1073 15 1123 15 1173 15 Resulting Austenite Formed (%) Start of Soaking End of Soaking 77 100 61 100 54 100 Austenite Formed at the end of Soaking (%) 32 83 92 99 Table 2. Heating Phase Parameters (Input and Output) Input Parameters Thermal Conditions 1 2 3 4 5 6 7 Heating Rate (K/s) 1 5 25 5 5 5 5 Soaking Temperature (T) 1173 1173 1173 1023 1073 1123 1173 Output Soaking Time (minutes) 10 15 15 15 15 15 15 Austenite Formed (%) 100 100 100 32 83 92 99 Forming In order to avoid cooling of the part before forming, the blank must be transferred as quickly as possible from the furnace to the press. Furthermore, forming must be completed before the beginning of the martensite transformation. Therefore, a fast tool closing and forming process are the precondition for a successful process control. After forming, the part is quenched in the closed tool, which is cooled by water ducts to transfer the heat out of the tool system. In order to avoid the quenching of the blank between the blank holder and the die during the forming process, most of the hot stamping tool systems work with a distance blank holder. Quenching After the forming of the heated blank in the austenitic temperature range, the part is quenched in the closed tool until the part microstructure is fully martensitic. A cooling rate of more than 27 K/s is necessary for a full martensite microstructure of 22MnB5. Martensite evolution leads to an increase of the flow stress. The transformation from austenite (FCC) into martensite (BCT) causes an increase in volume, which influences the stress distribution during quenching. For the accurate prediction of the resulting material properties, the volume fraction of different phases, the residual stresses, and the distortion of the workpiece after cooling, a complete description/analysis of the transformation behavior is needed [10]. References [1]. Li N., Lin J., Balint D. S., Dean T. A., 2016. “Experimental Characterization of the Effects of Thermal Conditions on Austenite Formation for Hot Stamping of Boron Steel”. Journal of Materials Processing Technology 231, pp: 254-264. [2]. Reed A. C., Akbay T., Shen Z., Robinson J. M., Root J. H., 1998. “Determination of Re-Austenitization Kinetics in a Fe-0.4C Steel using Dilatometry and Neutron Diffraction”. Materials Science and Engineering A 256, pp: 152-165. [3]. Schmidt E. D., Damm E. B., Sridhar S., 2007. “A Study of Diffusion-and-Interface-Controlled Migration of the Austenite/Ferrite Front during Austenitization of a Case-Hardened Alloy Steel”. Metallurgical and Materials Transactions A 38(4), pp: 698-715. [4]. Lechler J., Merklein M., 2008. “Hot Stamping of Ultra Strength Steels as a Key Technology for Lightweight Construction”. In: Materials Science and Technology (MS&T), Pittsburgh, Pennsylvania, pp. 1698-1709. [5]. Lechler J., 2009. Grundlegende Untersuchungen zur Berchreibung und Modellierung des Werkstoffverhaltens von presshärtbaren BorManganstählen. Dr.-Ing. Dissertation, LFT, University of Erlangen-Nuremberg. [6]. Savran V. I., 2009. Austenite Formation in C-Mn Steel. In: Materials Science and Technology. The Delft University of Technology. [7]. Huang J., Poole W. J., Militzer M., 2004. “Austenite Formation during Intercritical Annealing”. Metallurgical and Materials Transactions A 35(11), pp: 3363-3375. [8]. Cai J., 2011. Modeling of Phase Transformation in Hot Stamping of Boron Steel. Mechanical Engineering. Imperial College London. [9]. Liu F., Sommer F., Bos C., Mittemeijer E. J., 2007. “Analysis of Solid State Phase Transformation Kinetics: Models and Recipes”. International materials Reviews 52(4), pp. 193-212. [10]. Neubauer R., Hübner K., Wicke T., 2008. “Thermo-Mechanically Coupled Analysis: The Next Step in Sheet Metal Forming Simulation”. In: 1st International Conference on Hot Sheet Metal Forming of High Performance Steel, Kassel, Germany, pp. 275-283. [11].