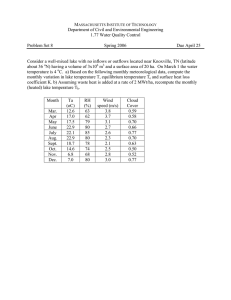
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/316024552 Lakes Nyos and Monoun Gas Disasters (Cameroon)—Limnic Eruptions Caused by Excessive Accumulation of Magmatic CO2 in Crater Lakes Article · April 2017 DOI: 10.5047/gems.2017.00101.0001 CITATIONS READS 35 4,260 1 author: Minoru Kusakabe University of Toyama 251 PUBLICATIONS 6,670 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Did CAMP magmatism extend to Cameroon? View project Satreps NyMo View project All content following this page was uploaded by Minoru Kusakabe on 09 August 2019. The user has requested enhancement of the downloaded file. GEochemistry Monograph Series, Vol. 1, No. 1, pp. 1–50 (2017) www.terrapub.co.jp/onlinemonographs/gems/ Lakes Nyos and Monoun Gas Disasters (Cameroon)—Limnic Eruptions Caused by Excessive Accumulation of Magmatic CO2 in Crater Lakes Minoru Kusakabe Department of Environmental Biology and Chemistry University of Toyama 3190 Gofuku, Toyama 930-8555, Japan e-mail: mhk2314@gmail.com Citation: Kusakabe, M. (2017) Lakes Nyos and Monoun gas disasters (Cameroon)—Limnic eruptions caused by excessive accumulation of magmatic CO2 in crater lakes. GEochem. Monogr. Ser. 1, 1–50, doi:10.5047/gems.2017.00101.0001. Abstract This is a review paper on the Lakes Nyos and Monoun gas disasters that took place in the mid-1980s in Cameroon, and on their related geochemistry. The paper describes: (i) the gas disasters (the event and testimonies); (ii) the unusual geochemical characters of the lakes, i.e., strong stratification with high concentrations of dissolved CO 2; (iii) the evolution of the CO 2 content in the lakes during pre- and syn-degassing; (iv) the noble gas signatures and their implications; (v) a review of models of a limnic eruption; (vi) a revision of a spontaneous eruption hypothesis that explains the cyclic nature of a limnic eruption (Kusakabe 2015); (vii) a brief review of the origin of the Cameroon Volcanic Line (CVL) and the geochemistry of CVL magmas; (viii) a brief review of other CO 2rich lakes in the world; and (ix) concluding remarks. Degassing of the two lakes has been successful. Most of the dissolved CO 2 has been removed from Lake Monoun, resulting in the stoppage of the degassing system. However, the CO2 content in the lake started to increase in recent years due to the continuing supply of gas from the underlying magma, indicating the necessity of the continuous removal of gas from the lake. Lake Nyos will attain the same situation in several years when degassing will stop. Thus, a continuation of scientific monitoring of the lakes is essential. Since the transfer to Cameroonian scientists of monitoring techniques, including analytical equipment necessary for the monitoring, has been effected through the SATREPS project (Japan’s Official Development Aid), the responsibility is now theirs, and it is strongly hoped that the lake monitoring, the rehabilitation of displaced people, and the setting up of an infrastructure for them, etc., will be carried out by the Cameroonian Government and local scientists. 1. Introduction Volatiles in the deep interior of the Earth are brought to the surface mainly by volcanic activity. In terms of the present-day global carbon cycle, the CO2 discharge from subaerial volcanism including the passive discharge from the craters or flanks of volcanoes, is the major non-anthropogenic contributor to atmospheric CO2 (e.g., Kerrick, 2001; Gerlach, 2011). The passive © 2017 TERRAPUB, Tokyo. All rights reserved. doi:10.5047/gems.2017.00101.0001 ISSN: 2432-8804 Received on December 5, 2015 Accepted on May 11, 2016 Online published on April 7, 2017 Keywords • Cameroon • Lakes Nyos and Monoun • gas disaster • crater lakes • magmatic CO2 • limnic eruption • disaster mitigation • degassing • Cameroon Volcanic Line • SATREPS degassing of CO2 is the quiet discharge of gas often derived from a magmatic source, with varying degrees of contamination by crustal or biological CO2. Crater lakes usually sit on top of volcanic conduits and act as condensers or traps for magmatic volatiles. The Lake Nyos gas disaster in 1986, and a similar event in 1984 at Lake Monoun, both in Cameroon, Central Africa, resulted from an excessive accumulation of magmatic CO2 in the bottom layers of the lakes. These volcanic 2 M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 Fig. 1. Location of Lakes Nyos and Monoun (red circles) and volcanoes along the Cameroon Volcanic Line (solid black) in Cameroon, Central Africa. Modified from figure 1 of Environmental Monitoring and Assessment Journal, Hydrogeochemistry of surface- and groundwater in the vicinity of Lake Monoun, West Cameroon: Approach from multivariate statistical analysis and stable isotopic characterization, 2015, Kamtchueng, B. T., Fantong, W. Y., Takounjou, A. F., Tiodjio, E. R., Kusakabe, M., Mvondo, J. O., Zhang, J., Ohba, T., Tanyileke, G., Hell, J. V. and Ueda, A. „ Springer International Publishing Switzerland 2015 with permission of Springer. crater lakes are considered to be the sites of passive degassing of CO 2 . On 26th August, 1986, a large amount of CO2 was suddenly released from Lake Nyos that asphyxiated 1746 people, and an unaccountable number of cattle, living near the lake (Sigvaldason, 1989). A very similar gas event took place in August 1984 at Lake Monoun, with 37 casualties (Sigurdsson et al., 1987). Lake Monoun is located only 100 km south-east of Lake Nyos (Fig. 1). A term “limnic eruption” was coined by J.-C. Sabroux to describe a gas outburst from a lake (Halbwachs et al., 2004), and will be used in this review. Given that this type of gas disaster had not been previously recorded (Sigurdsson, 1987a), the Lakes Monoun and Nyos events attracted a great deal of attention, not only from the media but also from a disaster science perspective. At that time, nobody imagined that the lakes had accumulated so much lethal gas and that the gas was released into the atmosphere without any precursor. Subsequent geochemical investigations revealed that the gas was CO2 that originated from magma and had accumulated passively in the deep part of these lakes. The physicochemical characteristics of the lakes are unique and have evolved with time, even after the gas release, due to the continuing supply of magmatic CO2. In the present paper, issues related to these gas discharges are reviewed in the following sections; (III) what happened at the time of the Lakes Nyos and Monoun gas disasters?; (IV) pre- and syn-degassing chemical evolution of the lakes; (V) possible causes of the disasters, the models and the repetitive nature of a limnic eruption. In relation to the recurrence prevention of a limnic eruption, a bilateral scientific project between Japan and Cameroon called SATREPSNyMo was carried out during 2011 and 2016, and is outlined in Section 5. The upper 40 m of Lake Nyos is bounded on the north by a narrow dam of poorly consolidated pyroclastic doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 3 Fig. 2. (a) Victims near Lake (Stager and Suau, 1987). Reproduced with permission of Helimission (www.helimission.org). (b) Dead cow by the lake (photo taken by the author). rocks. This dam is being affected by back erosion. A warning was given that the collapse of the dam could cause a flood that would affect inhabited areas over a 220 km distance (Lockwood et al., 1988). An accurate estimation of the rate of back erosion of the dam is critical for the safety of people living downstream. Thus, the age of the dam formation (or Nyos maar formation) has been hotly debated using different age determination techniques. Recent progress on the age of the dam is briefly reviewed in Section 6. Thirty nine crater lakes including Lakes Nyos and Monoun and numerous soda springs are located along the Cameroon Volcanic Line (CVL). An understanding of the origin and the geochemistry of CVL magmas is essential. These subjects are reviewed in Section 6, which constitutes the basis on which CO2 accumulation in these lakes is scientifically interpreted. We also need to understand why CO2 becomes enriched in magmatic volatiles as they leave the magma. The Lakes Nyos and Monoun events have stimulated geochemical interest in other CO2-rich volcanic lakes in the world for their gas hazard potential. This is reviewed in Section 7. 2. Gas disasters at lakes Nyos and Monoun, Cameroon 2-1. Cameroon: Location and physiography Cameroon is a country in Central Africa located between 2–13∞N latitude, and 8–16∞E longitude (Fig. 1). It is bounded by 6 countries: Chad to the northeast, Nigeria to the west, Central African Republic to the east, Equatorial Guinea, Gabon and Congo to the south. Cameroon can be divided into ten major ecological regions. These regions are classified under four re- gional units which are differentiated by their geography, climate and vegetation characteristics as follows: (1) The Sudano-Sahelian zone in the North is composed of the Mandara mountains, Diamaré plains and the Benue Valley. (2) The savanna zone is composed of the Adamawa highlands, the Tikar plain, the low land savanna of the Center and East regions, and the highland of the West and Northwest regions. (3) The tropical forest zone is composed of the degraded forests of the Central and Littoral regions, and the tropical rainforests of the Southwest and East regions. (4) The coastal and marine zone spreads along the Gulf of Guinea. The country’s economy is driven by agro-industry in the coastal, central and southern zones (Molua and Lambi, 2006). Because of the above geographic characteristics, its wide range of climatic types, and its cultural diversity, Cameroon is often nicknamed “Africa in miniature”. The population of Cameroon is estimated to be ~23 million as of January 2015 (http:/ /countrymeters.info/en/Cameroon). According to the Demographics of Cameroon (http://en.wikipedia.org/ wiki/Demographics_of_Cameroon), the country comprises an estimated 250 distinct ethnic groups, which may be classified into five large regional-cultural divisions: (1) the western highlanders (Semi-Bantu or grassfielders), including the Bamileke, Bamoun, and many smaller Tikar groups in the Northwest (~38% of the total population); (2) the coastal tropical forest peoples, including the Bassa, Duala (or Douala), and many smaller groups in the Southwest (12%); (3) the southern tropical forest peoples, including the BetiPahuin with subgroups called Bulu, Fang, Maka, Njem, and Bakapygmies (18%); (4) the predominantly Islamic peoples of the northern semi-arid regions (the Sahel) and central highlands, including the Fulani (or Fulbe) (14%); and (5) the “Kirdi”, non-Islamic, or recently doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. 4 M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 Fig. 3. An aerial view of Lake Nyos taken 10 days after the limnic eruption (photo taken by the author). Debris of vegetation washed away from the shore was floating on the reddened lake surface. Islamic, peoples of the northern desert and central highlands (18%). Since people of different ethnic groups speak different languages, French and English, inherited from colonialism, are used for mutual communication, although they retain their original languages. 2-2. Cameroon lakes There are at least 39 lakes of volcanic origin that are distributed along the CVL (Kling, 1988; Issa et al., 2014a). Lake Nyos in the Northwest Region of Cameroon (06∞26¢ N and 10∞17¢ E) is a meromictic volcanic crater lake with a N-S length of ~2.0 km, EW length of ~1.2 km, surface area of 1.58 km2, and a maximum depth of 209.5 m. It lies in the Oku volcanic field along the CVL, and was formed by a basaltic phreato-magmatic eruption (Lockwood and Rubin, 1989). The age of the lake, which has been a topic of controversy, will be described later (Subsection 6-1). Lake Monoun in the West region of Cameroon lies at 05∞35¢ N and 10∞35¢ E, and is also a meromictic volcanic crater lake with a NEE-SWW length of ~1.6 km, a maximum NW-SE width of ~0.7 km, a surface area of 0.31 km2, and a maximum depth of 99 m. It belongs to the Oku volcanic center along the CVL. The age of the lake is unknown. 2-3. Lake Nyos disaster: The event and testimonies Unusual news raced around the world in late August 1986. The first news that reached Japan reported that 40 local people had been killed by a “poisonous” gas released from a volcanic lake (Lake Nyos) in Cameroon. The number of casualties increased to ~1200 in a later report. In response to a request for international support by the Government of the Republic of Cameroon, the Japan International Cooperation Agency (JICA) under the Ministry of Foreign Affairs of Japan sent a Japan Disaster Relief Team (JDR) to the site. I was asked to join the team as a volcanic gas expert. It was my first visit to Cameroon. The JDR team arrived at Douala on 28 August, 1986. A few days later the team was taken to Lake Nyos by helicopter because of poor road conditions and heavy rains in the Nyos area. We were shocked by the terrible scenes we witnessed (Fig. 2), and the reddened surface water of the lake (Fig. 3), which increased our anxiety concerning the cause(s) of the disaster. Since the main purpose of JDR was to provide relief supplies and medical care to the refugees, we made an initial cursory scientific survey during this first visit. There was no indication of the direct involvement of volcanic gases as initially suggested, for we did not find any trace of acid gases like SO2, H 2S and HCl, which are major components of high-temperature volcanic gases. Later reports indicated that the cause of the deaths was CO2 gas released from Lake Nyos on the evening of 21 August, 1986, and that the gas killed 1746 people and ~8000 livestock by asphyxiation (Kling et al., 1987; Kusakabe et al., 1989). Exactly 2 years prior to the Lake Nyos disaster, there had occurred a very similar gas release from Lake Monoun on 15 August, 1984, that killed 37 people also by asphyxiation by CO2 gas released from the lake. These extremely unusual disasters had never been recorded before, and therefore constituted a new type of natural disaster (Sigurdsson, 1987a). August 21, 1986, was a Thursday, and a market day at the Nyos village. Local people were selling and exchanging their agricultural products and articles for daily use. At the time when the catastrophe occurred (8~9 p.m.), people must have been relaxed and chat- doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 ting at home, or drinking beer outside. Some people may have already been in bed. Such a peaceful situation was disrupted by a sudden release of lethal gas from Lake Nyos. It was indeed a nightmare. It is obvious that the local people did not understand what happened. From the testimonies collected later from survivors by journalists and researchers, the event may have proceeded as follows. Some people heard faint rumbles and noises like a car coming from a distance. They went out of the house to look around, and then felt a tepid breeze with a smell of rotten eggs or gun powder. Most people fell down, lost consciousness and died (Fig. 2). At Nyos village, where 1200 people lived at that time, only a few people (4~6) survived. The survivors were stunned to find that they had lost most of their family members, relatives and friends. Sigurdsson (1987b) noted that “some survivors of the disaster attributed it to the wrath of their dead tribal chief, who, on his deathbed in 1983, ordered that his best cattle be driven off the sheer cliffs above Lake Nyos as a sacrifice to the spirit of the lake, Mami-Water. But the chief’s family failed to honor his last wish, and many today believe that the 1986 calamity was an expression of the chief’s posthumous displeasure”. Sigurdsson (1987b) also described that, four days before the lethal event, local herdsmen noticed unusual bubbling on the lake’s surface, which prompted twenty five of them to move to a distant village. There were also unconfirmed reports claiming the emission of foaming water and vapor from the lake two to three weeks earlier. At about 4:00 p.m. on August 21, nearby herdsmen heard strange bubbling sounds and observed a slight emission of vapor from the lake. At about 8:00 p.m., villagers in Cha, a village about 7 km northwest of the lake, heard two loud noises, followed by three rumbles at about 9:00 p.m., when activity built up to the climactic disaster. According to Aramaki et al. (1987) who interviewed Mallam Jae, a local farmer who lived at a place 120 m higher than the lake surface, the gas explosion took place at about 8:30 p.m. on August 21 and continued until 1 a.m. the next day. This account of the time at which the events took place may be reliable, since Mallam Jae was wearing a nice wrist watch. Initially, Mallam Jae heard sounds like a murmur followed by detonations. He also felt tremors and a smell of gun powder. The next morning he found the lake quite unusual. Le Guern et al. (1992) published details of interviews with some local people who spoke in pidgin English (which was translated into English) about what they saw. Observations by local people included: (1) Minor upwelling of hydrothermal waters from the bottom of Lake Nyos on August 20, one day before the event. (2) A small explosion that took place at 4:00 p.m. followed by a major explosion between 8:00 and 8:30 p.m. on August 21. (3) A water jet accompanied by white illuminations. (4) A detonation was heard in 5 Fig. 4. Photograph showing a white cloud still remaining along the valley downstream of Lake Nyos. Nyos village is seen at the bottom. The photo was taken 2 days after the eruption by a helicopter pilot carrying a Catholic mission (supplied by G. Tanyileke). the village at 11:00 p.m. (5) Minor events with an upwelling of hydrothermal water and gas occurred in Lakes Nyos and Njupi, a small and shallow lake 2 km east of Lake Nyos (Chevrier, 1990). (6) White cloud was seen during the catastrophe on August 21. Based on these testimonies and observations, Le Guern et al. (1992) preferred the interpretation that the Lake Nyos catastrophe was caused by the input of hot hydrothermal fluids containing CO2 into the lake and surrounding area. Their interpretation seemed to have been influenced by their experience at Dieng volcano (Indonesia) where CO2 gas, originating from a phreatic eruption of the volcano, killed 142 local people who were fleeing from the site (Le Guern et al., 1982). However, there is a view that the anecdotal evidence (such as “the smell of rotten eggs and gun powder, rumbling noise heard at distance”, etc.), collected soon after the disaster through interviews with local survivors by journalists and scientists, should be interpreted with care because stories told by local people may have been tailored to give answers to please the visiting interviewers (Freeth, 1990). The author adopts this view and believes that the phreatic hypothesis did not have a firm scientific basis, because this interpretation of the events was largely based on the testimonies and anecdotal evidence. In March 1987, a Cameroon Government and doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. 6 M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 Fig. 5. Distribution of localities where victims were found around Lake Nyos (red circles), and estimated flow paths of the gas (arrows). Modified from Sigurdsson (1987a). UNESCO-sponsored international conference on the Lake Nyos Disaster was held in Yaoundé, the capital of Cameroon. More than 200 scientists participated and presented the results of their initial studies on the geological, geochemical, physical, medical and socio-anthropological aspects of the disaster (Sigvaldason, 1989). Regarding the cause of the gas burst, there was a sharp confrontation between a group of scientists who believed that the lake played a key role in the accumulation of the CO2 which was subsequently released (this interpretation was later named “limnic eruption hypothesis”) and another group of scientists who believed that the cause of the Nyos catastrophe was due to a volcanic (phreatic) eruption from the bottom of the lake (volcanological or phreatic hypothesis) (Tazieff, 1989; Barberi et al., 1989; Le Guern et al., 1992). Disagreement between the two scientific views resulted in a compromise of the resolutions of the Yaoundé Conference (Sigvaldason, 1989), and encouraged the need for follow-up investigations, which clearly indicated a steady supply of magmatic CO2 from the lake bottom and its accumulation in the lake. This gave strong support to the limnic eruption hypothesis (Kling et al., 2005; Kusakabe et al., 2000, 2008). This hypothesis will be described in detail in Sections 3 and 4. The gas released from Lake Nyos was almost pure CO2 (Kling et al., 1987; Kusakabe et al., 1989). Since CO2 gas is 1.5 times denser than air at room temperature, and since it may have been cooled due to adiabatic expansion when released from the pressurized deep part of the lake, the density of the gas was likely to have been significantly greater than that of the ambient atmosphere. This would have facilitated its flow along low-lying areas, such as valleys, before mixing with air. Costa and Chiodini (2015) simulated the gas flow, using a computer code TWODEE-2, for 4 different scenarios that considered different gas masses and fluxes from Lake Nyos in 1986. The simulations, indicating how far and fast the cloud dispersed after the limnic eruption, are useful for making up a hazard map of the area. Figure 4 is a photograph taken two days after the eruption by a Helimission helicopter pilot (G. Tanyileke, pers. commun.) and shows that the white cloud was still present along a valley downstream of the lake. Figure 5 (modified from Sigurdsson et al., 1987a) shows the gas flow path estimated from the distribution of victims around Lake Nyos. The gas cloud traveled more than 20 km, asphyxiating people on its way before dissipating into air. The number of victims was 1200 at Nyos village, 300 at Cha village and 52 at Subum village. More than 8000 cattle were also killed. Survivors were evacuated to 7 resettlement camps, namely, Kimbi, Buabua, Kumfoutu, Yemge, Ipalim, Esu and Upkwa (around Lake Wum). As of July 2015, these people were still cut off from the general population, as neither the national radio, electricity grid, nor television signals reached them. Since the victims were asphyxiated almost instantly, the oxygen concentration in the gas must have been extremely low compared to normal the atmosphere, or the CO2 concentration was very high. Table 1 shows the effect of some gases on human health (Kusakabe et al., 1989). Mammals, including human beings, live on a normal atmosphere that contains 21 vol % of O2. If air is breathed containing less than half of this normal air concentration of O2, a coma, fainting, cyanosis, syncope, respiratory arrest, and ultimately, cardiac arrest can result. If we breathe air containing high concentrations of CO2 (e.g., >10 vol %), a coma, and eventually, death can result. Some survivors of the Lake Nyos disaster were found to suffer from pulmonary edema, respiratory distress, conjunctivitis, and skin lesions or “burns” (Baxter et al., 1989). The skin burns were taken by the phreatic hypothesis supporters as evidence that the gas was at a high temperature and contained some acidic, corrosive components, such as SO2 (which turned to sulfuric acid later) and HCl that are commonly contained in high-temperature volcanic gases. This interpretation was highly unlikely, since vegetation, and clothes on the victims stayed intact and no appreciable level of sulfur and chlorine components were found in the lake water (Kusakabe et al., 1989). The medical interpretation of the skin damage or blisters was that the body’s metabolic rate was drastically reduced in a state of deep coma, inducing a severely restricted circulation of blood. As a consequence, the capillary vessels of the skin lacked circulation, resulting in necrosis and the development of skin lesions on approximately 5% of the patients (Baxter et al., 1989). doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 7 Table 1. Effect of some gases on human health*. Concentration in atmosphere Stage Response and symptoms O2 (%) 21 16-12 14-9 10-6 <6 1 2 3 4 5 Normal Lowered concentration, headache Disorientation, unstable gait, headache, nausea, vomiting, facial pallor, somnolence Coma, fainting, damage of central nervous system, cyanosis, convulsion Syncope, coma, bradypnea, respiratory arrest, cardiac arrest CO2 (%) 0.04 1.5 5 10 >40 1 2 3 4 5 Normal Changes in physiological ranges (techypnea etc.) Shortness of breath, headache, coma Coma in 10-15 min. exposure Sudden death *Reproduced from table 6 of J. Volcanol. Geotherm. Res. 39, Kusakabe, M., Ohsumi, T. and Aramaki, S., The Lake Nyos gas disaster: chemical and isotopic evidence in waters and dissolved gases from three Cameroonian crater lakes, Nyos, Monoun and Wum, 167–185, Copyright 1989, with permission from Elsevier. 2-4. Lake Monoun disaster Lake Monoun experienced a gas burst on 15 August, 1984, that killed 37 people by asphyxiation. A reconstruction of the event (Sigurdsson et al., 1987) showed that at almost midnight of that day, people in Njindoun, a village about 5 km north of the lake, heard a loud noise in the vicinity of the lake. They informed the nearby police early next morning. A policeman together with a medical doctor went to the site where they saw a whitish, smoke-like cloud that covered the ground to a height of ~3 m. Since they became nauseous and dizzy, they left the site and moved to Njindoun village. After the cloud dissipated, they came back to the site and found dead people lying on the road. The victims had skin lesions or blisters. Clothes were not affected. Domestic and wild animals were also found dead. Altogether 37 people were killed by this event. A survivor described the smell of the gas cloud as “sulfurous like a car battery”. It was found in a later survey that vegetation at the east end of the lake was flattened, indicating that the water wave locally reached up to 5 m high, and that the color of the lake water changed to a reddish brown. From the above statements, the Lake Monoun event was very similar to the Lake Nyos event. For this reason, it seems appropriate to describe and compare, at the same time, the results of the geochemical surveys made after the gas bursts at both lakes. 2-5. Unusual geochemical characters of Lakes Nyos and Monoun A scientific survey of Lake Monoun was undertaken by the Office of Foreign Disaster Assistance (USAID) several months after the gas burst, upon a request by the Cameroonian Government (Sigurdsson et al., 1987). They found unusual chemical characteristics. Waters below 50 m were anoxic, dominated by high Fe2+ (~200 mg/l) and HCO3– (~1000 mg/1), and supersaturated with siderite, a major component of the crater floor sediments. The unusually high Fe2+ levels were attributed to the reduction of laterite-derived ferric iron that was gradually brought into the lake as loess and in river input. Sulfur compounds were below detection limits in both water and gas. Table 2 shows the chemical composition of Lake Monoun water samples collected between 27 February and 16 March, 1985 (Sigurdsson et al., 1987). It includes data for samples collected in 1986 (Kusakabe et al., 1989) and 1993 (Kusakabe et al., 2008). Analysis of the 1985 and 1986 samples showed lower gas and ionic contents than the original solution. This was interpreted to be due to (i) loss of CO2 from the waters during retrieval of the Niskin sampler from the lake, (ii) loss of CO2 from waters collected in the sample containers, and/or (iii) oxidation and precipitation of iron prior to the analysis. During the early stages of investigation of the Lakes Monoun and Nyos disasters, these problems highlighted the difficulty of sampling and the analysis of CO2-rich waters. The data for the 1993 samples were more reliable (Kusakabe et al., 2008). Based on the data obtained in 1985, however, Sigurdsson et al. (1987) had come to the important conclusion that accumulation of CO2(aq) in the lake was attributed to the long-term emission of magmatic CO2(aq) from vents within the crater, which led to a gradual build-up of CO2(aq) and HCO3– in the lake, i.e., an essential concept of the “limnic eruption hypothesis”. It is noted that the manuscript of the paper by Sigurdsson et al. doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 8 Table 2. Representative analyses of water samples collected in 1985, 1986 and 1993 from Lake Monoun. Na+ mg/l K+ mg/l NH4+ mg/l Mg 2+ mg/l Ca2+ mg/l Fe2+ mg/l 6.9 5.8 6.3 6.4 6.1 6.3 6.4 6.0 5.9 9 17 25 24 24 25 24 26 26 2.2 4.7 5.6 5.7 5.7 5.8 5.9 5.5 5.5 <0.1 6.2 15 13 15 18 13 15 12 6.0 22 29 30 30 30 30 29 30 8.7 20 41 42 41 43 42 42 41 <0.02 0.03 200 220 170 260 190 290 190 October 1986*2 0 15 25 50 75 95 æ æ æ æ æ æ 11 14 17 17 21 22 3 4.2 5.3 5.1 7.2 7.2 æ 6 11 17 26 26 4.2 13 17 13 22 23 4 10 12 10 18 19 1.7 110 190 340 540 590 January 1993*3 10 20 30 40 50 55 75 90 95 100 6.58 6.46 6.00 5.91 5.80 5.60 5.58 5.60 5.66 5.72 13 15 18 19 21 23 24 24 25 26 3.5 4.3 4.6 5.0 5.3 5.5 5.8 5.4 5.9 7.4 10 12 18 19 22 28 28 30 37 39 8 15 24 24 23 27 27 28 29 29 13 21 30 33 38 46 46 48 50 51 100 259 464 505 533 641 646 682 804 918 Depth m February 1985*1 0 15 61 90 90 90 90 90 90 pH SO42mg/l Cl mg/l SiO2 mg/l HCO3mg/l CO2(aq) mg/l <1 <1 <1 <1 <1 <1 <1 <1 <1 <1 1.8 3.2 3.4 3.3 3.2 3 3.4 3.2 19 40 35 45 50 48 41 50 51 88 265 1050 1050 775 805 805 1000 885 26 952 1086 890 1311 851 680 2112 2357 0.1 0.3 0.2 0.4 0.4 0.2 0.4 1.0 1.4 2.5 2.5 2.6 17 40 37 38 42 44 57 421 657 1087 1520 1660 16 202 542 2859 2385 2922 0.1 0.1 0.1 0.1 0.1 0.1 0.1 0.1 0.1 0.5 1.1 1.5 1.5 1.6 1.8 2.3 2.4 2.4 2.6 2.8 34 58 86 91 95 110 114 114 123 129 82 105 1352 1448 1546 1823 1862 1961 2272 2523 4 18 1809 2271 3217 6760 6848 6813 6778 6029 * 1Sigurdsson et al. (1987). * 2Reproduced from table 2 of J. Volcanol. Geotherm. Res. 39, Kusakabe, M., Ohsumi, T. and Aramaki, S., The Lake Nyos gas disaster: chemical and isotopic evidence in waters and dissolved gases from three Cameroonian crater lakes, Nyos, Monoun and Wum, 167–185, Copyright 1989, with permission from Elsevier. * 3Reproduced from table 1 in Kusakabe et al. (2008). Numbers in italics were calculated assuming carbonate equilinria. (1987) had been prepared prior to the 1987 International Conference on the Lake Nyos disaster in Yaoundé, so the American team who started an initial scientific survey at Lake Nyos must have had a general idea of the cause of the disaster. Lake Nyos also has unusual chemical and physical characteristics similar to Lake Monoun. Dissolved species are overwhelmingly dominated by CO 2(aq) followed by HCO3–, Fe2+, Mg2+, Ca2+, SiO2, NH4+, Na+, K+ in decreasing order. The concentration of the dissolved species in the water column increases with depth with maximum values reached at the bottom (210 m). Follow-up studies of Lakes Nyos and Monoun clearly indicated that the CO2 content in the lakes was increasing at an unusually high rate for a geological phenom- enon (Evans et al., 1993; Kusakabe et al., 2000). This situation led scientists working on Lakes Nyos and Monoun to warn of the possible recurrence of a limnic eruption in the near future and to recommend the artificial removal of dissolved gases from the lakes (Freeth et al., 1990; Tietze, 1992; Kling et al., 1994; Kusakabe et al., 2000). To achieve this goal, the Nyos-Monoun Degassing Program (NMDP) was set up by scientists who were deeply involved in the disaster mitigation issues of the limnic eruption. After experimental degassing at Lake Monoun (Halbwachs et al., 1993) and Lake Nyos (Halbwachs and Sabroux, 2001), a permanent degassing apparatus was installed at Lake Nyos in 2001 and at Lake Monoun in 2003 under the NMDP, funded by the U.S. Office of Foreign Disaster Assist- doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 9 Fig. 6. Fountains from the degassing pipes. The fountain heights were 45 m at Lake Nyos, February 2001 (a) and 8 m at Lake Monoun, January 2004 (b). The tapping depth of the pipes was 203 m and 73 m, respectively. ance (USAID) and the Cameroonian Government. Controlled degassing is continuing successfully at both lakes. Figure 6 shows the amazingly beautiful fountains in the initial phase of degassing at Lakes Nyos and Monoun, a 45 m high fountain at Lake Nyos (Feb. 2001) and a 8 m high fountain at Lake Monoun (Jan. 2004). The degassing system and the construction of the degassing pipes have been described in Halbwachs et al. (1993, 2004). There was concern that artificial degassing might trigger another limnic eruption, since degassing could bring deep water to the surface, which will become cooler due to adiabatic cooling, and therefore may sink and destabilize the lake (e.g., Freeth, 1994). However, numerical modeling of the evolution of CO2 in the lake under different input conditions (McCord and Schladow, 1998; Kusakabe et al., 2000; Schmid et al., 2003, 2006) suggested that destabilization of the water column due to controlled degassing could not pose an immediate threat from a “man-made” limnic eruption. However, the possibility of thermal instability of the water column between 50–70 m, which could become a trigger for a limnic eruption, was suggested by Schmid et al. (2004), for they found double-diffusive convection at that depth range. In agreement with the results of the numerical modeling, the observed chemical structure of the lakes after the initiation of the controlled degassing operation indicated that a stable stratification was established, which remained basically the same as the predegassing situations at both lakes (Kling et al., 2005; Kusakabe et al., 2008). As stated above, the chemical and physical structure of Lakes Nyos and Monoun evolved steadily with time until the early 2000s when degassing started. After the initiation of gas removal, the lake structure was obviously affected by degassing. For this reason, the evolution is better described separately as “pre-degassing” and “syn-degassing”. 3. Pre- and syn-degassing evolution of Lakes Nyos and Monoun 3-1. Pre-degassing evolution The first scientific reports on the 1986 Lake Nyos gas disaster were published by Freeth and Kay (1987), Kling et al. (1987) and Tietze (1987). Of these, Kling et al. (1987), which is easily accessible, gave the most comprehensive results of the initial survey of the disaster, which included the geology of the region, the geochemistry of water and gas from the lake, and the pathology of hospitalized people and victims. They concluded that (i) the gas released was CO2 that had been stored in the lake’s hypolimnion (bottom layer), (ii) the victims died of CO2 asphyxiation, (iii) CO2 was derived from magmatic sources, and (iv) there was no direct volcanic activity involved. Kusakabe et al. (1989) reached similar conclusions on the basis of water chemistry and carbon and noble gas isotopic compositions of the gases dissolved in Lakes Nyos and Monoun. They noted that the H2S concentration in the released gas must have been far below a lethal level, a point that precluded the phreatic eruption hypothesis (see above). The same authors also reported the first petrochemical data of ejecta around Lakes Nyos and Monoun, which indicated that the lavas were transi- doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. 10 M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 Fig. 7. Profiles of electric conductivity normalized at 25∞C (abbreviated as C25) at Lake Monoun, January 2003 (left) and Lake Nyos, January 2001 (right). The water column of each lake can be divided into 4 layers, each separated by a chemocline. Reproduced from Fig. 1 and Fig. 7 of Kusakabe et al. (2008). Fig. 8. Evolution of pre-degassing temperature profiles at Lake Monoun (a) (1986–2003) and (b) Lake Nyos (1986–2001). Reproduced from figures 4 and 9 of Kusakabe et al. (2008), but colored. tional to slightly alkaline in composition (see below). A detailed temporal variation of the chemical structure of Lakes Nyos and Monoun since the limnic eruptions at both lakes was reported by Kusakabe et al. (2008). This paper presented the most comprehensive data set of chemical composition, conductivity, temperature, pH and CO2 profiles obtained from measurements taken almost every 2–3 years from 1986 to 2006, which enabled an evaluation of the evolution of CO2 in the lakes over a period of about 20 years and which encompassed pre- and syn-controlled degassing periods. The chemical structure of the lakes is best represented by a conductivity profile (Fig. 7). Both lakes have a similar chemical structure which is character- ized by four distinct layers. At Lake Monoun, layer I is the shallowest, is well-mixed, and contains low conductivity water. A sharp chemocline separates layers I and II at 23 m in January 2003. Layer II extends down to a 51 m depth, where a second chemocline develops. A well-mixed layer III continues down to ca. 85 m. Below this depth, conductivity increases steadily toward the bottom (layer IV). Lake Nyos has basically the same structure as Lake Monoun in January 2001: layer I is the shallowest, is well mixed, and contains low conductivity water. A sharp chemocline at about a 50 m depth separates layers I and II, the latter extending down to about a 180 m depth. A lower chemocline develops around this depth, below which a well-mixed doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 11 Fig. 9. Evolution of pre-degassing conductivity profiles at Lake Monoun (a) (1986–2003) and (b) Lake Nyos (1986–2001). Reproduced from figures 4 and 9 of Kusakabe et al. (2008), but colored. layer III continues down to ~203 m. Below this depth, conductivity sharply increases towards the bottom (layer IV). Pre-degassing temperature variations at Lakes Monoun (October 1986 to January 2003) and Nyos (November 1986 to January 2001) are shown in Figs. 8a,b, respectively, (reproduced from Kusakabe et al., 2008). Temperature profiles at Lake Monoun show a minimum at the 5–21 m range (layer I); followed by (i) an increasing temperature to about 23∞C down to the lower chemocline at depths of 50–63 m (layer II), (ii) constant values down to around 90 m (layer III), and (iii) a second increase to >23∞C towards the bottom (layer IV). It is worth noting that the temperature of the layer III water increased significantly between 1986 and 1999, and that, at the same time, layer III (thermally homogeneous zone) widened, forming a “shoulder” at a depth of 51 m. This widening suggests that warmer water was added to layer IV, and the profiles were pushed upward. A simple heat balance indicates that the heat accumulated in layers III and IV during 15 years (October 1986 to January 2003) is 7.8 ¥ 1012 J, supplying heat at an average rate of 5.1 ¥ 10 11 J/yr (~0.02 MW). The incremental upward movement of the lower thermocline (Fig. 8a) indicates the addition of water to layer IV, most likely from the bottom. If 4.1 ¥ 108 tons of water having a temperature of 27∞C were added, it would account for the heat accumulation during that period. Diffusive and conductive heat loss to layer II and above was not taken into consideration in this simple heat balance calculation, thus giving a minimum heat supply. Note that the rate of heat and water supply to layers III and IV initially ap- pears high judging from the change in the temperature profiles (Fig. 8a). Similar to Lake Monoun, the temperature of the Lake Nyos bottom water increased continuously after the limnic eruption in 1986 (Fig. 8b), indicating a heat input into the lake. The heat input to layers III and IV was reported to decrease from an initially high value of 0.93 MW (August 1986 to May 1987) to 0.43 MW (November 1986 to December 1988, Nojiri et al., 1993) to 0.32 MW (May 1987 to September 1990, Evans et al., 1993). Figure 9 shows the temporal change in the predegassing conductivity profiles at both lakes. As previously stated, Lake Monoun profiles have a “shoulder” between layers II and III. The shoulder became shallower and sharper, and layer III widened with time and its conductivity increased (Fig. 9a). Vertical conductivity profiles in layer III suggest that the layer is well mixed. The rise of the shoulder indicates an addition of recharge water from the bottom, pushing bottom water upward. This is consistent with the changes in the temperature (Fig. 8a). By combining the conductivity profiles from October 1986 to January 2003 (15 years) with the bathymetry used in Kling et al. (2005), we calculated an overall increase of 2.7 ¥ 10 3 tons of Total Dissolved Solids (TDS) in layers III and IV. This translates into an average annual TDS input rate of 1.7 ¥ 102 tons/yr. The sharp conductivity rise toward the bottom in layer IV may be related to dissolution and reduction of particles containing ferric compounds to release Fe2+ and HCO 3– in the sediments that are rich in organic material. The concentration of Fe2+, HCO3– and NH 4+ increased significantly with depth doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. 12 M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 Fig. 11. Comparison of the CO 2 concentrations at Lake Nyos measured by the pH method (solid curve) in March 1995, those obtained by the syringe technique (red open circles, November 1993) and the cylinder technique (blue open squares, April 1992, Evans et al., 1994). Dotted curves along the pH-based CO2 profile indicate possible errors due to an uncertainty in the pH measurement of ±0.05. Modified from figure 2 of J. Volcanol. Geotherm. Res. 97, Kusakabe, M., Tanyileke, G., McCord, S. A. and Schladow, S. G., Recent pH and CO 2 profiles at Lakes Nyos and Monoun, Cameroon: implications for the degassing strategy and its numerical simulation, 241–260, Copyright 2000, with permission from Elsevier. Fig. 10. Schematic presentation of the “MK sampler”. Reprinted from figure 2 of J. Volcanol. Geotherm. Res. 97, Kusakabe, M., Tanyileke, G., McCord, S. A. and Schladow, S. G., Recent pH and CO 2 profiles at Lakes Nyos and Monoun, Cameroon: implications for the degassing strategy and its numerical simulation, 241–260, Copyright 2000, with permission from Elsevier. only in layer IV whereas the other ions such as Na+, K+, Mg2+, Ca2+ showed a steady increase with depth (Kusakabe et al., 2008). At Lake Nyos, shallow water in November 1986 had a higher conductivity, even at about 7 m (Fig. 9b), than that in later years, indicating that deep, TDS-rich water was brought to the surface during the limnic eruption, the effect still remaining 3 months after the limnic eruption. This upper chemocline in November 1986 deepened gradually with time down to 30 m in 1988, 47 m in 1993 and 50 m in 2001. Conductivity profiles at mid-depths (70–160 m) stayed almost unchanged for 15 years after the eruption, suggesting that transport of dissolved chemical species through layer II was limited. The conductivity in layers III and IV (170–210 m) increased notably with time (Fig. 9b). In January 2001, the conductivity profile between 185 m and 202 m became steep, with an associated slight reduction of earlier high conductivity in layer IV, indicating the initiation of mixing in the deepest zone. This tendency had started in 1998, although the 1998 profile is partially obscured behind the 2001 profile in Fig. 9b. From the depth of 205 m to the bottom, the conductivity increased sharply. This trend is the same as observed at the bottom water of Lake Monoun. The pre-degassing increase of the conductivity in layers III and IV from November 1986 to January 2001 (14 years) corresponds to an increase of 7700 tons of TDS, with the average annual input of 540 ton/yr. Initially, the input rate was relatively high, but later decreased by at least a factor of two as shown by the close spacing of the conductivity profiles (Fig. 9b). This temporal trend was similar to that of the water temperature. Before describing the temporal variation of CO2 profiles in the lakes, it may be worthwhile mentioning the analytical methods used to determine the dissolved CO2. As stated before, during the early days of our observations, we encountered difficulties in measuring CO2 from deep water. The partial pressure of dissolved gases in Lake Nyos was so high (~1.1 MPa in 1990, Evans et al., 1993) that we could not use a Niskin water sampler to collect water and gases, since the lid of the sampler was forced open before retrieval due to doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 13 Fig. 12. (a) Cylinder sampler used by Evans et al. (1993). A pre-evacuated cylinder is deployed to a desired depth, and a check valve is opened to sample water. (b) Gas pressure probe used by Evans et al. (1993). Dissolved gas molecules except water diffuse through the membrane unit consisting of multiple silicone rubber tubing. The total gas pressure inside the collection chamber is measured at the surface. Reprinted from figures 3 and 4 of Appl. Geochem. 8, Evans, W. C., Kling, G. W., Tuttle, M. L., Tanyileke, G. and White, L. D., Gas buildup in Lake Nyos, Cameroon: The recharge process and its consequences, 207–221, Copyright 1993, with permission from Elsevier. the exsolution of high-pressure dissolved gases. This difficulty was partially solved by attaching a gas bag to the Niskin sampler, which enabled us to collect water samples (Kusakabe et al., 1989), but it was still difficult to measure the dissolved CO2 accurately. To overcome this difficulty we developed a new method called the “the MK or syringe method” (Kusakabe et al., 2000). The sampler in the MK method is schematically shown in Fig. 10. In this technique, the total dissolved carbonate species (H2CO3 + HCO3– + CO32–) at a given depth is fixed in situ in a 50-ml plastic syringe containing concentrated (5 M) KOH solution. The total carbonate concentration in the alkaline solution is determined later in the laboratory by a classical micro-diffusion method (Conway, 1958). Subtraction of the HCO 3– concentration and the blank carbonate in the KOH solution from the total carbonate concentration gives the H 2CO3 (or CO2,aq) concentration. Since the total carbonate species dissolved in Lakes Nyos and Monoun are essentially controlled by car- bonate equilibria, it is possible to determine the H2CO3 (or CO2,aq) concentration from pH values (measured by CTD) if the HCO3– concentration at a corresponding depth is known (Kusakabe et al., 2000). The HCO3– concentration is closely related to the electric conductivity, so we can calculate the concentration of H2CO3 (or CO2,aq) of a water column using the CTD data. It is a big advantage that we can obtain a continuous CO2 profile, although very careful calibration of the pH sensor is an important prerequisite. This method is called “the pH method”. Figure 11 compares CO2 profiles at Lake Nyos in 1995 obtained by the pH and syringe methods. In the figure, the results obtained by the “cylinder method” are included. In the cylinder method (Fig. 12a), deep water was sucked into a remotely-operated evacuated stainless steel cylinder and the exsolved total CO2 was later analyzed in the laboratory (Evans et al., 1993). They also introduced an interesting device called a “gas-probe” (Fig. 12b) with which the total gas pressure at a given depth of a water doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. 14 M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 Fig. 13. Analytical system for measuring CO2 concentrations in gassy lakes (copied from Yoshida et al., 2010). Two-phase flow (gas and water) from a plastic hose deployed to a desired depth in the lake is introduced into a separator and a gas flow meter. The amount of water accumulated in the separator and the volume of gas that goes through the flow meter in a given time are measured, from which the CO2 concentration is calculated. Fig. 14. Evolution of pre-degassing CO 2 profiles at (a) Lake Monoun (1986–2003) and (b) Lake Nyos (1986–2001). The saturation of CO2 in water at 25∞C is shown by a dashed line. Reproduced from figures 4 and 9 of Kusakabe et al. (2008), but colored. Note that the CO2 concentrations at a depth of ~55 m in 2001–2003 at Lake Monoun are close to saturation. column was measured. Figure 11 shows the satisfactory agreement between the CO2 concentration obtained by the different methods. Recently, “the plastic hose method” (Yoshida et al., 2010) for CO2 determination has also been used. This is based on a self-gas-lifting principle in a plastic hose that is deployed into the deep water of the lake. A mixture of gas and water spouting from the hose is separated into liquid and gas phases by using a plastic sepa- rator. The liquid phase accumulates in the separator and is collected as the water sample, while the dry gas flows through a volumetric gas meter to measure the gas volume at the sampling time (Fig. 13). A gas sample collected directly from the dry gas line is perfectly free from air contamination which is essential for noble gas analysis (Nagao et al., 2010). A similar method has been reported by Tassi et al. (2009) for gas collection from Lake Kivu. doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 15 Table 3. Change with time in CO2 content at Lakes Monoun and Nyos during the last 28 years. Date Time aftereruption year Lake Monoun: Pre-degassing October 1986 November 1993 April 1996 November 1999 December 2001 January 2003 2.17 9.25 11.67 15.25 17.33 18.42 Lake Monoun: During-degassing January 2004 19.42 January 2005 20.42 June 2006 21.92 January 2007 22.42 December 2007 23.33 January 2009 24.42 January 2011 26.42 March 2012 27.59 March 2013 28.59 Apr 2014 29.84 Total CO2 giga mol CO2 below layer II giga mol CO2 accumulation rate giga-mol/yr CO2 removal rate giga-mol/yr æ æ æ æ æ æ æ æ æ æ 0.098 (2003-2007) æ 0.005 (2009-2011) æ æ æ 0.38 0.38 0.53 0.59 0.53 0.59 0.60 0.61 0.61 0.60 0.61 0.61 æ æ æ æ æ 0.0084 (1993-2003) 0.53 0.42 0.43 0.22 0.11 0.071 0.041 0.066 0.074 0.079 0.52 0.42 0.42 0.21 0.10 0.055 0.036 0.051 0.059 0.065 æ æ æ æ æ æ æ æ æ 0.0048 (2011-2014) Lake Nyos: Pre-degassing November 1986 December 1988 November 1993 April 1998 November 1999 January 2001 0.17 2.33 7.25 11.67 13.25 14.42 13.1 13.3 12.9 13.3 13.6 14.1 14.4 14.8 13.6 14.0 14.0 14.6 Lake Nyos: During-degassing December 2001 January 2003 January 2004 January 2005 January 2006 January 2007 January 2009 January 2011 March 2012 March 2013 March 2014 15.33 16.42 17.42 18.42 19.42 20.42 22.42 24.42 25.59 26.59 27.59 14.2 13.1 13.2 12.3 11.8 11.6 11.2 10.0 7.8 6.6 5.9 14.0 13.1 13.0 12.6 11.7 11.4 11.1 9.7 7.7 6.5 5.8 æ æ æ æ æ 0.12 (1986-2001) æ æ æ æ æ æ æ æ æ æ æ æ æ æ æ æ æ æ æ æ æ æ æ æ 0.46 (2001-2011) æ æ 1.2 (2011-2014) This table was revised from table 1 in Kusakabe et al. (2008). CO 2 removal rate was calculated using the CO2 content below layer II during the period shown in parentheses. Data after 2011 were supplied by T. Ohba. More recently, a new and simple method of measuring the total CO2 (CO 2,aq and HCO3–) has been developed by Saiki et al. (2016). This method is based on a linear relationship between the total CO2 concentration and the sound velocity in lake water. Temporal pre-degassing CO2(aq) variations at Lakes Monoun and Nyos are shown in Fig. 14. Although no data were available in 1986 at Lake Monoun, the 1986 profile was estimated from later CO2-conductivity relationships (Kusakabe et al., 2008). Figure 14 shows that CO2(aq) concentrations in deep lake water were around 130 mmol/kg in layers III and IV, with the CO2 doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. 16 M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 Fig. 15. Evolution of syn-degassing CO2 profiles at (a) Lake Monoun (2003–2014) and (b) Lake Nyos (2001–2014). The saturation of CO 2 in water at 25∞C is shown by a dashed line. Recent data were added to figures 5 and 11 of Kusakabe et al. (2008), and the figures were colored. Note that the CO2 concentrations in 2012, 2013 and 2014 in deep water at Lake Monoun have increased, indicating a re-buildup of gas. CO2 profiles at Lake Nyos have steadily subsided. The highest CO 2 concentration at the bottom water in 2014 was reduced to ~150 mmol/kg. See text for details. shoulder at a depth of ~63 m in 1986. The CO2(aq) profiles evolved with time, especially from 1986 to 1993. The thickness of layers III and IV expanded with time, supporting the hypothesis that CO2-rich recharge fluid was added from the bottom. In December 2001 and January 2003, the CO2 shoulder at a 58 m depth (157 mmol/kg) was very close to the CO2 saturation concentration (Duan and Sun, 2003) at a depth of 50 m. Considering that the rate at which the shoulder was rising was about 1 m/yr, saturation at a 58 m depth could be reached in several years. The formation of CO 2 bubbles which could induce a limnic eruption (Kozono et al., 2016) would have occurred soon after 2003 at Lake Monoun if no degassing operation had been undertaken. Figure 14b shows the temporal variation of CO2(aq) profiles between November 1986 and January 2001 at Lake Nyos. The general features of Fig. 14b are summarized as: (i) CO2(aq) concentration was lowest in the early days after the explosion; (ii) there was little change with time at mid-depths (~50 m to 150 m); (iii) the greatest change took place at a depth of >170 m, where the CO2(aq) concentration at a given depth increased significantly with time; and (iv) the CO2(aq) concentration at the bottom-most water was almost constant, near 350 mmol/kg since 1999. The constancy of the bottom water CO2(aq) concentration was confirmed by later syn-degassing measurements. The change in the bottom water is likely caused by the gradual addition of recharge fluid having a CO 2(aq) concentration of ~350 mmol/kg. The CO2(aq) content of the lake was calculated by integrating CO2(aq) profiles over the water column below layer II using the bathymetry in Kling et al. (2005) under the assumption that the horizontal distribution of CO2 was uniform, as deduced from the conductivity distribution (see figure 8 of Kusakabe et al., 2008) and that CO2 loss through the upper chemocline was negligible. Thus, the CO2 accumulation rate can be regarded as the CO 2 recharge rate. Accumulation of CO2 in layer II, and in deeper layers, is tabulated in Table 3 for Lakes Monoun and Nyos. Considering that the CO2(aq) profile in October 1986 at Lake Monoun was estimated in an indirect way (Kusakabe et al., 2008), the overall rate of CO2 accumulation below the upper chemocline was calculated using the 1993 to 2003 profiles. The change in CO2(aq) content below layer II in the main basin for the pre-degassing period (1993 to 2003) was ~80 (=610–530) Mmol, with a CO2 recharge rate of 8.4 Mmol/yr. Almost the same recharge rate of 8.2 Mmol/yr was reported by Kling et al. (2005) using their own data obtained between 1992 and 2003 for Lake Monoun. At Lake Nyos, the CO2(aq) content below layer II steadily increased by ~1.7 Gmol until January 2001, when permanent degassing started. The increase in the CO2(aq) content can be translated into the CO2 recharge doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 17 Fig. 16. The reduced height of fountains from degassing pipes at Lakes Nyos and Monoun. Three degassing pipes were in operation at Lake Nyos as of March 2014 (c). Gas self-lift capability was lost in January 2009 at Lake Monoun, leaving a weak bubbly flow from the neck of the pipe (f). rate, which was 0.12 Gmol CO 2/yr between November 1986 and January 2001 (Table 3). Again, the rate is in good agreement with the value of 0.13 Gmol CO2/ yr given by Kling et al. (2005). 3-2. Syn-degassing evolution of CO2 content and future prospect The CO2(aq) profiles between 2001 and 2011 at both lakes during syn-degassing are shown in Fig. 15. Generally speaking, degassing went smoothly, as illustrated by the steady subsidence of the CO 2(aq) profiles. This resulted in a lowering of the fountain height at both lakes (Fig. 16). The subsidence has continued up to the present time; however, a buildup of CO2 has resumed recently at Lake Monoun (see below). The overall shape of the profiles did not change with degassing, showing that only bottom water and dissolved CO2(aq) were removed without causing any effect on the stratification of the lake water. At Lake Monoun (Fig. 15a), the highest CO 2(aq) concentration at the bottom decreased to 80 mmol/kg, and the thickness of layer III reduced to ~20 m in 2009, and further reduced to ~70 mmol/kg and ~15 m, respectively, in 2011 (Kusakabe et al., 2011). In 2009, two of three degassing pipes stopped working completely, and the other pipe issued only a weak bubbly flow (Fig. 16f). Thus, it can be said that the degassing pipes at Lake Monoun have almost lost their gas self-lift capability. Moreover, recent observations (2011–2014) show that CO2 concentrations below 80 m and the layer III thickness are increasing (Fig. 15a), clearly indicating that natural CO2 recharge into Lake Monoun still continues. This confirms our prediction that CO2 re-buildup is inevitable if lake degassing stops. On the basis of a geochemical study on the generation of CO2 in the Nyos mantle, Aka (2015) has suggested that CO2 will be continuously supplied into the lake for a geologically long time in the future. This view may also apply to Lake Monoun. In order to avoid gas re-buildup and to make the lake continualy safe, Yoshida et al. (2010) suggested continuously removing the bottom water that contains the CO2 at a significantly high concentration. The installation of such a bottom water removal system was undertaken at Lake Monoun in December 2013 (Yoshida et al., 2016), and details of the system are doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. 18 M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 Fig. 17. (a) Change with time in the CO2 content at Lakes Monoun (a) and Nyos (b). Modified from figures 6 and 12 of Kusakabe et al. (2008) to which recent data were added. The blue and red circles denote pre-degassing and syn-degassing evolution, respectively. Note that the CO2 content at Lake Monoun started to rise after 2011 at a rate of ~4.8 Mmol/yr, approximately half of the natural CO2 recharge rate of 8.4 Mmol/yr estimated from the pre-degassing data. The degassing rate at Lake Nyos was accelerated after installation of 3 pipes. The CO2 content at Lake Nyos will attain a minimum in several years time. described in Section 5. At Lake Nyos (Fig. 15b), CO2(aq) profiles subsided steadily until January 2011, resulting in a very thin layer III by that time. As two more degassing pipes with a greater diameter (25.7 cm I.D.) were installed in December 2011–March 2012, the degassing rate was greatly enhanced, resulting in a rapid decrease of CO2 concentration in deep water in the subsequent years (2011–2014). We can expect that most of the CO2-rich bottom water will disappear from Lake Nyos in several years from now and that the gas self-lift capability will be lost as in Lake Monoun. Using CO2(aq) profiles and lake bathymetry (Kling et al., 2005), the amount of CO2(aq) dissolved below the upper chemocline (layers II, III and IV) was calculated as a function of time since the limnic eruption at both lakes (Fig. 17). The amount of dissolved CO2 in Lake Monoun (Fig. 17a) increased steadily at a rate of 8.4 Mmol/yr, reaching a maximum value of 610 Mmol in January 2003, shortly before the degassing opera- tion started. Degassing was effective, reducing the amount of dissolved CO2 at a mean gas removal rate of 98 Mmol/yr between January 2003 and December 2007 (see Table 3). This rate is approximately 12 times greater than the natural recharge rate as shown by the sharp slope (Fig. 17a). The installation of two additional pipes in April 2006 accelerated the gas removal rate. In January 2009, the system had almost lost its gas self-lifting capability, resulting in a reduction of gas removal rate to only 5 Mmol/yr in January 2011 (Table 3), although a very weak flow of bubbly water from one of the three pipes was still visible. At that time, the amount of CO2(aq) dissolved in deep water was 55 Mmol, or only 9% of the maximum value observed in 2003 (Kusakabe, 2015). Based on these observations, it can be said that Lake Monoun has been made safe. However, the tailing-off of the CO 2 content after 2009 (Fig. 17a) implied that a buildup of CO2 is inevitable at Lake Monoun if the natural recharge of CO 2 continues at the previously estimated doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 19 Fig. 18. dD-d 18O values of monthly collected rain waters (dark asterisks) and ground waters (circles and triangles) sampled in the vicinity of Lake Nyos are shown (Kamtchueng et al., 2015a). Those of Lake Nyos waters (Nagao et al., 2010) are also included. All values are consistent with the global meteoric water line of Rozanski et al. (1993), although the Lake Nyos waters are plotted slightly upper-right of the cluster. It may suggest that the lake water is not recharged by recent groundwater. rate. Indeed, the re-buildup of CO2 became obvious after March 2012 (Fig. 17a). Using the data between 2010 and 2014, the rate of gas re-buildup is calculated to be ~4.8 Mmol/yr which is about half of the CO2 recharge rate of 8.4 Mmol/yr calculated from the predegassing data (Table 3), although we need to accumulate more recent data for a more reliable determination of the rate of gas re-buildup. The current amount of total dissolved CO2 in Lake Monoun is 79 Mmol (as of April 2014) which is ~13% of the maximum predegassing value recorded in 2003. It may take another ~100 years to reach the pre-degassing situation if the current rate of gas re-buildup remains unchanged. For these reasons, it is essential to continue monitoring the lake on a regular basis. The evolution of CO2 content over time since 1986 at Lake Nyos is shown in Fig. 17b. The gas removal rate by a single pipe (0.46 Gmol/yr) is about four times greater than the natural recharge rate of 0.12 Gmol/yr. At this removal rate, however, it would take another 20 years or so to remove all the gas from the lake. Fortunately, using funds from the Government of Cameroon and UNDP, two additional degassing pipes were installed in early 2011. Since pipes with a greater diameter (25.7 cm) were used and the water intake depth was increased to close to the bottom for the additional pipes, the rate of gas removal greatly increased to 1.2 Gmol/y (Table 3). It is hoped that most of the remaining gas will be removed within the next 5 years or so. At the last stage of the degassing operation, the rate of gas removal will decrease due to a lower CO2 concentration at the intake depth. This will lead to gas re-buildup, as we have seen at Lake Monoun. Thus, a system to pump up CO2-rich bottom water needs to be set up after the current degassing system has lost its gas self-lifting capability. 3-3. Hydrogen, oxygen, carbon, and noble gas isotopic signatures The hydrogen and oxygen isotopic ratios of Lake Nyos waters were first reported by Kling et al. (1987). The data for Lakes Nyos, Monoun and Wum (a crater lake near Lake Nyos) were later added by Kusakabe et al. (1989) and Nagao et al. (2010). The isotopic determination was intended to find any input of volcanic gases into Lake Nyos, for volcanic gases are usually characterized by high d18O signatures, but the data did not indicate any volcanic input. The dD and d 18O values from Lake Nyos plot close to the Global Meteoric Water Line (Craig, 1961; Rozanski et al., 1993) when combined with the data for rain water, groundwater, and surface water recently collected in the Lake Nyos catchment area as shown in Fig. 18, although the lake doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. 20 M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 Fig. 19. Relationship between 3He/ 4He (in R atm) and 40Ar/ 36Ar ratios of waters in Lakes Nyos and Monoun. R atm is a 3He/4He ratio of sample relative to that of air (=1.4 ¥ 10–6). The plots show a mixing of magmatic gases and the atmosphere (green cross). See text for a discussion. Data from Nagao et al. (2010). water is slightly more enriched in heavy isotopes than the ground and surface waters (Kamtchueng et al., 2015a). Noble gas information was used to constrain the origin of CO2 dissolved in the lakes, for noble gases do not react with rocks and waters on the way to the Earth’s surface. Figure 19 shows the relationship between 3He/4He and 40Ar/36Ar ratios for Lakes Nyos and Monoun gases in 1999. Coupled with the d 13C values of –3.3 ~ –3.4‰ (relative to Vienna Pee Dee Belemnite, VPDB) for Lake Nyos and –6.8‰ for Lake Monoun, the helium and argon isotopic ratios suggest a strong affinity of the dissolved gases with a magmatic source (e.g., Kusakabe and Sano, 1992). Nagao et al. (2010) reported more precise data using air-contamination free samples. The 3He/ 4He ratios in the gases in the Lake Nyos deep waters are ~5.7 Ratm, where Ratm is the atmospheric ratio of 1.4 ¥ 10–6. The Lake Nyos 3He/4He ratios are lower than the typical mantle values of 7~9 Ratm for depleted mantle producing Mid-Oceanic Ridge basalts (MORBs) (Graham, 2002). The reasons why the 3He/4He ratios of Lake Nyos are lower than the mantle values are related to the sub-lithospheric structure beneath the Cameroon Volcanic Line (CVL) as discussed later (Section 6). Halliday et al. (1988) reported variations in the radiogenic isotopic ratios (Pb, Nd, and Sr) of volcanic rocks along the CVL, where the highest 206Pb/ 204Pb and 208Pb/ 204Pb ratios were found at the oceanic and continental boundary. Barfod et al. (1999), Aka (2000) and Aka et al. (2004) published a detailed study of noble gases in basalts and xenoliths from CVL volcanic rocks, showing a symmetrical distribution of 3He/ 4He ratios along the CVL (Fig. 20). The lowest 3He/4He ratios (4.5 ¥ 10–6 or ~3 Ratm) were found at Etinde, a small volcano next to Mt. Cameroon, located at the oceanic and continental boundary. The 3He/ 4 He ratios become close to the MORB values (7~9 Ratm) as we go away from the oceanic and continental boundary towards both ends (Aka et al., 2004). This symmetric isotopic variation was explained as reflecting the geochemical characteristics of the mantle, or the continental lithosphere underneath the boundary which is of a HIMU character (Halliday et al., 1988). HIMU is geochemical jargon for “highm” with m defined as the ratio of 238U/204Pb. HIMU mantle is characterized by an enrichment in U and Th, the parent elements of radiogenic Pb and He. Melts derived from this HIMU mantle are postulated to have been emplaced beneath the oceanic and continental boundary. Thus, rocks at the oceanic and continental boundary are high in 206Pb/ 204Pb and low in 3He/ 4He ratios. Mineral separates from rocks around Lake Nyos (clinopyroxene and amphibole in xenolith) have 3He/ 4 He ratios of 6.7~7.0 R atm (Aka et al., 2004), slightly lower than the typical MORB values, implying a small degree of the HIMU character of the magma source beneath Lake Nyos. The deep water of the lake has even lower values of doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 21 Fig. 20. Symmetrical distribution of 3He/4He and 206Pb/204Pb ratios of rocks along the CVL as a function of distance from Annobon. This distribution suggests a contribution of the HIMU mantle for magma genesis in the oceanic and continental boundary volcanoes (Halliday et al., 1988; Aka et al., 2004). Oceanic sector volcanoes are Annobon (AN), São Tomé (ST) and Principé (PP). Ocean/continent boundary volcanoes are Bioko (BK), Etinde (ETD) and Mount Cameroon (MC). Continental sector volcanoes are Manengouba (MB), Bambouto (BT), Oku (OK), and Ngaoundere (ND). Fig. 21. Sampling water and gas using the “Flute de Pan” (a). Water and gas gushing out of 11 plastic hoses (O.D. of 15 mm) with different intake depths were collected (b). ~5.7 Ratm, as was stated above. This low ratio may mean that He in deep water was originally derived from magma generated from a slightly HIMU-type mantle, but acquired radiogenic 4He on the way from the source magma to the sub-lacustrine fluid reservoir during the passage of the magmatic fluid through granitic basement rocks. Nagao et al. (2010) reported the distribution of isotopic ratios of not only He but also Ne, Ar, Kr, Xe and C in Lakes Nyos and Monoun waters collected at closely separated depths. They stressed the importance of samples that were free from air-contamination, because noble gas concentrations, especially those of Ar and Ne, are so low in gases exsolved from CO2-rich waters compared to air so that any samples exposed to the atmosphere during sampling, or storage in an improper way, are not good for analysis. Samples from Lake Nyos (January 2001) were collected using the “Flute de Pan” which had been deployed by the French scientific team. This consisted of 11 plastic hoses having an outside diameter of 15 mm with different intake depths (83–210 m) (Fig. 21). CO 2-rich gas spouting out of a given hose was collected in a glass bottle using an inverted funnel placed in a bucket. Although slight contamination of air dissolved in the water was still suspected to some extent, especially for heavy noble gases, this sampling method was found to doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. 22 M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 Fig. 22. Depth profiles of noble gas concentrations in water (10–6 ccSTP/gwater) measured in 2001 at Lake Monoun (a) and Lake Nyos (b). Noble gas concentrations in air saturated water (ASW) at 30∞C (table 2 in Kipfer et al., 2002) are also shown by arrows for comparison. Modified from figures 1 and 2 of Nagao et al. (2010). be promising. In the December 2001 sampling, a plastic hose method, essentially the same as the Flute de Pan method, was adopted. A single plastic hose (12 mm I.D.) was deployed initially to the bottom, followed by pulling it upward little by little to a desired depth. Exsolved CO2 gas was directly allowed to pass through a sampling bottle made of uranium glass that has a low He diffusivity. With this method, Nagao et al. (2010) were able to collect air-contamination free samples. This method was later modified to measure the total gas concentration on site (Yoshida et al., 2010). Figures 22a, b illustrate the profiles of He, Ne, Ar, Kr and Xe in water, measured in 2001, at Lake Monoun and Lake Nyos, respectively. Except for He, they show roughly constant concentrations with respect to depths below 80 m at Lake Nyos, and 50 m at Lake Monoun. The Ne, Ar, Kr and Xe concentrations are up to several times lower than those in air saturated water doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 23 Fig. 23. 3He/4He ratios as a function of depth at Lakes Nyos and Monoun in 1999 and 2001. Modified from figure 4 of Nagao et al. (2010). (ASW). Depth profiles of the 3He/ 4He ratio for Lakes Nyos and Monoun are presented in Fig. 23. The data published by earlier workers are in the same range (Kling et al., 1987; Sano et al., 1987, 1990; Kusakabe and Sano, 1992). Generally speaking, the 3He/4He ratios are almost constant in the depth range of 80–210 m at Lake Nyos, and 40–100 m at Lake Monoun. The 3 He/ 4He ratio approaches the atmospheric value in waters shallower than 80 m and 40 m for Lakes Nyos and Monoun, respectively. High 4He/ 20Ne ratios up to ~1500, as shown in Fig. 22, support the premise of magmatic gas input to the lake as inferred by high 3He/ 4 He ratios. Neon isotopic ratios are presented in Fig. 24. Compared to atmospheric Ne, small excesses of both the 20 Ne/22Ne and 21Ne/22Ne ratios are observed. Most data points for both lakes lie on the MORB line connecting atmospheric Ne and mantle Ne as reported by Staudacher and Allègre (1988). The data clearly indicate the presence of mantle Ne in the lakes, and are consistent with the conclusions of Barfod et al. (1999) and Aka et al. (2004) that the CVL mantle contains MORB-like Ne. Argon isotopic ratios are presented in Fig. 25. The 40 Ar/36Ar ratios for all samples are higher than the atmospheric value of 296, but much lower than the estimated value of >1650 for Ar in the upper mantle beneath the CVL (Barfod et al., 1999). This means that magmatic fluids containing mantle Ar mixed with atmospheric Ar on the way to the surface such as in a sub-lacustrine fluid reservoir. The contribution of man- tle Ar to the sub-lacustrine Ar may be less than 20% assuming that the mantle Ar has a 40Ar/ 36Ar ratio of >1650. This is consistent with the conclusion derived from the Ne signature (Fig. 24), although the contribution of mantle Ne to the sub-lacustrine Ne may be ~6%. At Lake Nyos, the highest 40Ar/ 36Ar ratio, of about 600, was found at the bottom (210 m). The 40Ar/ 36 Ar profile in January 2001 decreased gradually towards the surface approaching the atmospheric ratio, but it showed a zigzag pattern below the lower chemocline at ~180 m with a second maximum value of 480 at 190 m. The zigzag 40Ar/36Ar profile disappeared in December 2001 with consistent ratios around 530 below 190 m. This may have resulted from vertical mixing in this depth range caused by degassing, because water was pumped out by the degassing pipe from an intake depth of 203 m. A tendency for such homogenization was also observed in the water temperature and electric conductivity at the corresponding depths (Kusakabe et al., 2008), although they were less clear than the noble gas profiles. At Lake Monoun, the 40Ar/36Ar ratios were in a narrow range of about 470 between 60 m and 100 m (bottom) (Fig. 25). The ratios are lower than those in the deep waters of Lake Nyos, suggesting that the contribution of atmospheric Ar to the magma-originating gases at Lake Monoun is greater than that at Lake Nyos. The characteristic features of noble gases observed at Lake Nyos can be summarized as follows (Nagao et al., 2010): (i) Helium in the lake water derived originally from the mantle where 3He/ 4He ratios of ~7 Ratm doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. 24 M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 20 Ne/22Ne versus 21Ne/22Ne plot for samples collected in 2001 at Lakes Nyos and Monoun. The “mass fractionation line” indicates the isotopic trend of atmospheric Ne due to mass fractionation. The dashed line heading to MORB represents a mixing line between atmospheric Ne and Ne in MORB or the upper mantle (Ballentine et al., 2005). Modified from figure 5 of Nagao et al. (2010). Fig. 24. Fig. 25. Depth profiles of 40Ar/36Ar in Lakes Nyos and Monoun in 2001. Chemoclines were taken from Kusakabe et al. (2008). Modified from figure 6 of Nagao et al. (2010). are found in mantle xenoliths (Aka et al., 2004), but, on its way to the surface, approximately 20% of radiogenic 4He that accumulated in crustal rocks was admixed to give ratios of ~5.7 R atm, probably in the sublacustrine region. (ii) The observed 40Ar/36Ar ratios of 450–550 are also explained by the addition of atmospheric Ar (40Ar/ 36Ar = 296) carried by groundwater to mantle-originating Ar (40Ar/36Ar >1650, Barfod et al., 1999) on the way to the lakes. The most likely source of Ar to reduce the mantle 40Ar/36Ar ratio is atmospheric Ar-bearing groundwater. (iii) Ne in the lakes may be a mixture of atmospheric Ne and a small amount of MORB-like Ne from the mantle. The observed He, Ne and Ar isotopic ratios in lake waters can be best explained by mixing between two noble gas reservoirs, i.e., air dissolved groundwater and the mantle. It is doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 25 Fig. 26. (a) “Inflating” CO2 profiles in the deep water of Lake Nyos in the depth range of 160–210 m between 1986 and 2001. (b) 3He profile in the same depth range observed in 2001. Note the sharp maximum of 3He concentration at 188 m where a chemocline (dashed line) existed in 2001. Modified from figure 14 of Volcanic Lakes (Dmitri Rouwet, Bruce Christenson, Franco Tassi, Jean Vandemeulebrouck, eds.), Evolution of CO2 content in Lakes Nyos and Monoun, and sub-lacustrine CO2recharge system at Lake Nyos as envisaged from CO2/3He ratios and noble gas signatures, 2015, pp. 427–450, Kusakabe, M., „ Springer-Verlag Berlin Heidelberg 2015 with permission of Springer. conceivable that the mantle-derived gases with the addition of radiogenic 4He from crustal rocks and atmospheric Ar and Ne carried by groundwater are finally homogenized in the sub-lacustrine reservoir. Note that the contribution of atmospheric He to deep lake water, if any, is difficult to find, since the He concentration in deep lake water is more than 3 orders of magnitude higher than that in ASW, whereas the contribution of the other noble gases is more easily discernible because of their similar concentrations in deep lake water and ASW (see Fig. 22). As stated previously, the greatest chemical change in Lake Nyos took place at depths greater than 180 m. The CO2 profiles (1986–2001) in the depth range 160– 210 m are enlarged in Fig. 26a. This shows that the increase of CO2 concentration in the deep water of Lake Nyos after the 1986 limnic eruption resulted from widening of CO 2-rich water leading to the formation of a clear lower chemocline at the top of the CO2-rich water. The 3He concentration observed in 2001 in the same depth range was compared with the CO2 profiles (Fig. 26). The 3He profile was obtained from the 4He profile (Fig. 22) and the 3He/ 4He profile (Fig. 23). It should be noted that the 3He concentration below 160 m in January 2001 and December 2001 shows a sharp maximum at around 188 m with a concentration up to 9.1 ¥ 10–10 ccSTP/g-water (December 2001) (Fig. 26b). The 4He concentrations have a pattern very similar to those of 3He in the same depth range, because of the almost constant 3He/4He ratios (Fig. 23), although the 4 He concentrations are not graphically shown. Between 190 and 210 m, the 3He concentrations are nearly constant at ~5 ¥ 10–10 ccSTP/g-water. The 3He concentration gradually decreases as the depth decreases (layer II). The C/3He ratios of volcanic fluids have been widely used to constrain magma sources. The C/3He ratios of MORB glasses are shown to be fairly constant at 0.20 (±0.05) ¥ 1010, suggesting that the source region of MORB in the upper mantle has little variation in the C/3He ratio (Marty and Jambon, 1987). The ratios for volcanic gases from subduction volcanism, however, have been found to be significantly greater than the MORB values, i.e., 0.7 ¥ 1010 ~ 3 ¥ 10 10. These high ratios, coupled with d13C values, indicate the existence of recycled carbon (marine carbonates, slab carbonates and/or organic materials) in subduction zone magmas (Sano and Williams, 1996, and references therein). Figure 27 shows the C/ 3He ratios in the depth range 160–210 m in Lake Nyos. The C/3He ratios range from 0.5~1.7 (¥ 1010). These values are higher than the mantle values of ~0.2 ¥ 1010. It is interesting to note that the C/3He ratios in waters below the lower chemocline are significantly high at around 1.6 ¥ 1010, and sharply decrease to 0.5 ¥ 1010 above the lower chemocline. Thus, the behavior of CO2 and 3He are decoupled be- doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. 26 M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 Fig. 27. C/ 3He atomic ratios observed in the depth range of 160–210 m at Lake Nyos in 2001. The C/ 3He ratios were calculated from the CO2 and 3He profiles shown in Fig. 26. A clear difference is seen across the chemocline (dashed line). Modified from figure 15 of Volcanic Lakes (Dmitri Rouwet, Bruce Christenson, Franco Tassi, Jean Vandemeulebrouck, eds.), Evolution of CO2 content in Lakes Nyos and Monoun, and sub-lacustrine CO 2-recharge system at Lake Nyos as envisaged from CO2/3He ratios and noble gas signatures, 2015, pp. 427–450, Kusakabe, M., „ Springer-Verlag Berlin Heidelberg 2015 with permission of Springer. Fig. 28. (a) Change with time of the CO2/ 3He ratio in fumarolic gases from Mammoth Mountain in the Long Valley caldera, California (1988–1998). (b) Change with time of the He concentration in the same gases. Data of Sorey et al. (1998) were used. doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 27 Fig. 29. Schematic presentation of the sub-lacustrine fluid reservoir which is encircled by a green circle. The geological crosssection of Lake Nyos was taken from Lockwood and Rubin (1989). Blue arrows indicate the possible flow of groundwater, and red arrows indicate a magmatic fluid coming from the magma underneath. Noble gas and carbon isotopic ratios of respective reservoirs are shown. Modified from figure 16 of Volcanic Lakes (Dmitri Rouwet, Bruce Christenson, Franco Tassi, Jean Vandemeulebrouck, eds.), Evolution of CO2 content in Lakes Nyos and Monoun, and sub-lacustrine CO2-recharge system at Lake Nyos as envisaged from CO2/3He ratios and noble gas signatures, 2015, pp. 427–450, Kusakabe, M., „ Springer-Verlag Berlin Heidelberg 2015 with permission of Springer. low and above the chemocline. The cause(s) of the decoupling may be explained by the underplating of the recharge fluid from the bottom that is characterized by different C/3He ratios. By “underplating”, I meant that the recharge fluid is added to the bottommost water from beneath. It is possible that the ratio was low before the limnic eruption and high after the limnic eruption. At the time of the limnic eruption, the lake was not completely mixed, suggesting that deep water still contained a large fraction of “pre-eruption” water (Giggenbach, 1990; Tietze, 1992; Evans et al., 1994) which may have been proportionally higher in He and lower in CO 2 concentrations with the CO2/3He ratio of ~0.5 ¥ 1010. Recharge fluids entering the lake after the eruption may have a CO2/3He ratio of ~1.6 ¥ 10 10. This interpretation implies that the CO2/ 3He ratio in the recharge fluids may vary with time and has changed from low to high values with time. A change with time in the CO2/ 3He ratio has been observed in fumarolic gases from Mammoth Mountain in the Long Valley caldera, California, where “tree-kill” took place due to an anomalous discharge of magmatic CO2 into soils (Farrar et al., 1995; Sorey et al., 1998). This anomalous CO2 discharge was induced by an episode of shallow dyke intrusion beneath Mammoth Mountain in 1989–1990. The CO2/3He ratios of the fumarolic gases there changed from ~0.3 ¥ 10 10 to 1.6 ¥ 1010 in about 10 years (Fig. 28). The change was caused by a trend of decreasing He concentration and little change in the concentration of CO2, which is a major component of the gases. The 3He/ 4He ratios stayed at around 5.5 Ratm with a few exceptions. These observations indicate that the above geochemical parameters (CO2/ 3 He ratio and He concentration) that carry information about magmatic fluids can change within a geologically very short period of time, i.e., in the order of 10 doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. 28 M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 years, at a single volcanic system. Thus, it is conceivable that the decoupling of CO2 and 3He observed at Lake Nyos after the limnic eruption was caused by the addition of “recent” recharge fluids that were characterized by relatively low 3He concentrations. From the foregoing discussions based on noble gas signatures and C/3He ratios, we can envisage the sub-lacustrine CO2-recharge system at Lake Nyos to be as shown in Fig. 29. 4. Limnic eruption, models, triggers and c yclicity 4-1. Models Many hypotheses have been put forward to explain why the limnic eruption occurred. Sigurdsson et al. (1987) proposed that a landslide slumped into deep water, pushed CO2-rich water up and induced the 1984 limnic eruption at Lake Monoun. The same idea was also suggested for the 1986 Lake Nyos event (Kling et al., 1987). Tietze (1987) suggested supersaturation of dissolved CO2 just below the shallowest chemocline (~8 m depth in 1986) to be the main cause of the eruption. The strong density stratification of this layer worked as a lid for rising gases, inhibiting them from penetrating this density divide. The supersaturation that followed led to the exsolution of gases to form a fountain. This process was self-intensified and deeper water was steadily degassed in turn. Since the water from the fountain was cooler than the surface water, it sank around the fountain, forming a cylindrical “density wall”. This wall limited lake-wide exsolution of gases, leaving CO 2 dissolved in deep water (>150 m?) intact during the eruption. Assuming that Lake Nyos was isothermal and fully saturated with CO2, Kanari (1989) presented a fluid-dynamics model to explain how the limnic eruption proceeded. In his model, degassing started from the bottom but was confined to a limited area at the surface. Circulation of water was confined in small cells that stacked at various depths. According to this model, stratification within the lake was hardly affected. Kanari estimated that (i) the released gas volume (0.68 km3) was the difference between the saturation and the CO2 profile observed in 1986 by Kusakabe et al. (1989), (ii) the maximum height of the gas cloud was 110 m, and (iii) the speed of the gas cloud running down the valley was 19 m/s. However, later observations indicated that full CO2 saturation over the entire lake was unlikely (Kusakabe et al., 2008). Obviously, any degassing model depends on a knowledge of the pre-eruption distribution of CO2 in the lake. Evans et al. (1994) proposed a model based on a linear pre-eruption relationship between CO2 and TDS (total dissolved solids) at Lake Nyos using water chemistry, CTD measurements, gas analyses and tritium profiles obtained between 1987 and 1992. A linear relationship between tritium and TDS was interpreted to reflect the destruction of the pre-existing gradient at mid-depth during the eruption, suggesting that CO2 exsolved from deep water. In their model, the upper chemocline was placed at ~50 m depth, similar to the chemocline depth observed in January 2001, 14 years after the limnic eruption, and just prior to the initiation of artificial degassing (see Fig. 9). Some triggers, such as a combination of seasonal decline in the water column stability, landslide and/or seiche, pushed water upward at a layer around the chemocline to the CO2 saturation depth. Bubble formation then followed and relatively quiet degassing continued. A local reduction in the hydrostatic pressure beneath the release area created a rising column of shallow, slightly gassy water. This was followed by mixing with pre-release surface water (low TDS) to form the surface water that was observed soon after the limnic eruption. The base of the column became slowly deeper, bringing CO2-rich, more saline deep water upward. When the base of the column reached the deeper chemocline, below which CO2 and TDS concentrations were much higher, gas release became more violent and created wave damage along the lake shore such as the flattening of vegetation and the passing of water over an 80-m-high promontory in the southern part of the lake. The duration of this violent fountaining was short (<1 min), and the amount of CO2 released was estimated to be 6.3 Gmoles. This scenario is consistent with the testimonies of survivors. Giggenbach (1990) proposed that the gas release at Lake Nyos was triggered by a climatic factor. The descent of a parcel of unusually cold rain water (18.5∞C) pushed initially CO2-rich shallow water upward. The uplift of the CO 2-rich water above the saturation depth induced bubble formation which accelerated upward movement by a reduction of density, leading to the formation of a convecting water flow that entrained deeper, more CO2-enriched water, and, finally, to the limnic eruption. Less-dense degassed waters accumulated at the surface, making it difficult for deeper CO2rich water (>100 m) to reach the surface, thus terminating the eruption. Deep water CO2 was therefore left almost intact. The amount of CO2 released was estimated at 5.4 Gmoles. In contrast to the previous models for the cause of the limnic eruption, spontaneous exsolution of dissolved gases has been suggested by Kusakabe et al. (2008) and Kusakabe (2015). In this scenario, attention was paid to the pre-degassing evolution of dissolved CO2 at Lake Monoun (see Fig. 14a) which indicated that CO2(aq) profiles evolved with time and that CO2-rich layers below the lower chemocline (layers III and IV) widened due to the continuing recharge of CO2-charged fluid from beneath. Note that the CO2(aq) concentration in water below layer III was constant at ~150 mmol/kg. In January 2003, just prior to the initiation of the degassing operation, the CO2(aq) concen- doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 29 tration immediately below the chemocline at the boundary between layers II and III was very close to saturation. If no degassing operation were undertaken, the saturation of CO 2(aq) would have been attained at that depth in a short time (within several years), and bubble formation would have followed by additional input of the recharge fluid. Thus, at Lake Monoun another limnic eruption could have occurred spontaneously within several years after 2001, if any external trigger had been introduced to this critical situation. 4-2. Spontaneous eruption hypothesis The evolution of pre-degassing CO 2 profiles at Lake Monoun gives a clue to estimate a pre-eruptive CO2 profile at Lake Nyos. It is conceivable that it was similar in shape to the 2001–2003 profiles at Lake Monoun (see Fig. 14a). It is interesting to note that the CO2 profiles at the deep layer of Lake Nyos (>180 m in 1999 and 2001; Fig. 14b) was developing in a way similar to that observed at Lake Monoun. The thickness of CO2-rich water close to the bottom kept increasing after the 1986 eruption till 2001 due to the addition of the recharge fluid from beneath. The CO2(aq) concentration below 195 m reached 350 mmol/kg in 1999. This concentration remained unchanged until January 2011 down to the bottom (Kusakabe et al., 2008; Kusakabe, unpublished data). This observation suggests that the CO 2(aq) concentration of the recharge fluid is constant at ~350 mmol/kg. If no degassing was undertaken, and if the natural recharge of CO2 continued as before, the thickness of the bottom CO 2-rich water would have continued to increase, and the top level of the CO 2-rich layer could have eventually reached saturation at some shallower depth. This speculation is schematically presented in Fig. 30. In this model, the pre-eruption profile, shown as “Before 1986”, has a shoulder that touches the saturation curve at a depth of ~110 m. A limnic eruption would take place spontaneously, releasing the dissolved gases to the atmosphere, resulting in a CO2 profile shown as “November 1986” in Fig. 30 (process 1). The observed evolution of the CO2 concentration between November 1986 and January 2001 is shown as “process 2” in Fig. 30. If no degassing took place, and if the natural recharge of CO2 continued as before, the CO 2(aq) profile would have shifted upward following “process 3”, and would eventually have touched the saturation curve. Saturation is a necessary condition, but may not be a sufficient condition, for a limnic eruption to take place. Rising bubbles may re-dissolve in under-saturated water during ascent. However, if sufficient CO2 flux is given, the bubbles can reach the surface, possibly leading to a limnic eruption. Based on the above model, a numerical approach to the recurrence of a future limnic eruption was made by Kozono et al. (2016). They demonstrated that a plume of bubbles Fig. 30. A model of the spontaneous limnic eruption at Lake Nyos. An assumed pre-eruption CO2 profile is shown by red small open circles as “Before 1986”. After the eruption, the CO 2 profile turned to the post-eruption profile shown as “Nov. 1986” (process 1). It evolved to the January 2001 profile (blue) in 15 years (process 2). If the natural recharge continues, the January 2001 profile may “recover” the preeruption situation (process 3). Modified from figure 9 of Volcanic Lakes (Dmitri Rouwet, Bruce Christenson, Franco Tassi, Jean Vandemeulebrouck, eds.), Evolution of CO2 content in Lakes Nyos and Monoun, and sub-lacustrine CO2recharge system at Lake Nyos as envisaged from CO2/3He ratios and noble gas signatures, 2015, pp. 427–450, Kusakabe, M., „ Springer-Verlag Berlin Heidelberg 2015 with permission of Springer. generated from a growing CO2-saturated surface (the top of the “Before 1986” curve in Fig. 30) can reach the lake surface with a high flux of CO2, i.e., limnic eruption, if any external forcing triggers bubble formation at the growing CO2-saturated surface. The trigger may be an instability caused by double diffusive convection (Schmid et al., 2004), or a seiche near the CO2-saturated surface where the density gradient is strong. If our model is correct, the difference between the pre- and post-eruption profiles integrated over the lake volume gives the amount of gas released at the time of the eruption, which was calculated to be ~14 Gmol or 0.31 km3 (at STP). This value is greater than the estimate of 0.14 km3 by Evans et al. (1994) by a factor of ~2, but significantly smaller than earlier estimates (0.7~1 km3) by Faivre Pierret et al. (1992), and Kanari (1989). The estimated amount of CO2 released obviously depends on the assumptions involved. As long as the lake receives a continual natural recharge of CO2, limnic eruptions can occur repetitively (Tietze, 1992), but may not be regular as described in the model of doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. 30 M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 Chau et al. (1996), which considered a possible variation in the rate of the natural recharge of CO2. However, if the conceptual model shown in Fig. 30 is correct, it would take ~100 years to attain the pre-eruption CO2 level shown by the “Before 1986” curve starting from the curve “November 1986” assuming a constant CO2 recharge rate of 0.12 Gmol/year (process 3). 4-3. Repetitive nature of a limnic eruption The above model implies that the time of repetition of a limnic eruption is ~100 years. A possibility of cyclic gas bursts from lakes which are charged by a gas influx from the lake bottoms was also pointed out by Tietze (1992). He argued that dissolution of CO2(aq) will inevitably create a stratification of the lake because the density of CO2-containing water is higher than that of pure water, due to a small partial volume of dissolved CO2(aq) in water (Ohsumi et al., 1992), and that the stratification will limit upward gas transport, leading to an accumulation of the gas below the stratified layers. If limnic eruptions take place repetitively on a timescale of ~100 years, evidence of past eruptions might be found in geological records and local documents. Unfortunately, no geological evidence has been recorded, and no such written documents are known to exist in the Nyos-Monoun areas. However, Shanklin (1989, 1992, 2007) published interesting folklores that are common in the grassfields of western Cameroon; the Kom story and the Oku story. The folklores are suggestive of limnic eruptions that took place in the past. The following paragraphs (shown in italic) are reproduced from Shanklin (1992). The Kom Story Kom people were living at Bamessi (near Lake Nyos) as guests of the Fon (a ruler is called Fon in the Grassfields), but the Bamessi Fon was afraid the Kom were becoming too powerful and he devised a trick to rid himself of them: he suggested to the Kom Fon that since their young men were showing signs of their reigns, they each should build a house and entice the young men inside, then bar the doors and set the houses afire. But the wily Bamessi Fon built his house with two doors and so all the Bamessi men escaped, while all the Kom men died. Soon the Kom Fon discovered the trick and vowed revenge. First, he called his sister to him and told her of his plans. He would hang himself and Kom people were not to cut his body down, nor even go near it; instead, they were to watch and wait for the appearance of a python track that would lead them to their new home. Led by the Fon’s sister, the Kom people followed their Fon’s instructions precisely. After he hanged himself, his body fluids dripped down and formed a lake; the Kom people watched. Maggots from the Fon’s body fell into the lake and became fish; the Kom people watched. The people of Bamessi were delighted with the new lake and they in- formed their Fon, who proclaimed a day when they would all go into the lake to catch the fish. The day came and the Kom people watched the people of Bamessi assemble at the lakeside; then the Bamessi went into the lake to catch fish for their Fon. Then the Bamessi went back to catch fish for themselves. At that point, Kom people say, the lake “exploded”, then sank and disappeared, taking with it most of the Bamessi population. Thus was the Kom Fon’s curse fulfilled; the people of Bamessi were destroyed, leaving the enemy Fon with only a few retainers as he had left the Kom Fon when the two houses were burned. As they watched from the hills, the python trail appeared to the Kom people and they turned away to begin the long journey west, to the area they now occupy. The Oku Story At Oku there is a good-sized crater lake and Oku people say that at one time two groups were settled beside the lake. On the western slope were the Babanki or Kijem people and on the eastern slope were the Oku people. Each had their own Fon. There were many disputes between them, one being a disagreement as to which group owned Lake Oku. One day a stranger came and asked the Fon of Kijem for land on which to build a compound. The Kijem Fon was a disagreeable fellow and he refused to give land. The stranger then went to the Oku Fon, who gave him a building plot. But the stranger did not like the land that was given, so he went back to the Oku Fon and asked for a different plot. The Fon allocated him another, but again the stranger was not happy, so he returned to the Fon, asking for a different place. Once again, he was given a plot, and once again, he returned to complain about it. Finally the Oku Fon, seeing that the man would not be satisfied, told him to choose his own land. The man settled down beside Lake Oku, and, as it is said in Pidgin English, no one ever knew what he did there. (The implication is that the man had no visitors because he was a witch.) When the stranger died, the Kijem and Oku people went to celebrate his death, the Kijem people on their side of the lake and the Oku people on theirs. Both Fons were called to come into the lake (presumably by the now-dead stranger) and they did so, each entering from his side. They were then taken to the lake bottom and, soon after they disappeared, streaks of red (blood) began to appear on the Oku side. As they watched the red streaks come up, the Oku people thought their Fon was dead and they began to mourn for him. At the same time, there appeared in the distance a Fon dressed in fine new clothes, and the Kijem people began to cheer, believing their Fon was being returned to them, having been honored by the host with precious garments. But, in fact, it was the Oku Fon who was dressed in fine clothes and the Kijem Fon who had been slaughtered. The two groups returned to their homes, wondering what would come next. Soon after, the waters of Lake Oku left the lake doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 31 bed and destroyed most of the homes and people on the Kijem side; the remnant moved away from the lake, further west into the nearby Belo Valley. Oku people still elaborate annual sacrifices to the lake, which showed by its actions that it wished to belong to the Oku people. From that day to this, no red streaks have appeared in the lake. From the above stories we can find one common theme that “maleficent” water misbehaves in a spectacular way and sets in motion the migration of ethnic groups. I believe that this point indicates the occurrence of a limnic eruption of Lake Nyos in the past, although we cannot specify the date(s) and lake(s) of the past eruptions. 5. SATREPS-NyMo: A project to reduce the risk of another limnic eruption The Science and Technology Research Partnership for Sustainable Development (abbreviated as SATREPS) is a program for joint research cooperation between Japan and developing countries for resolving global issues, e.g., environment, energy, natural disaster prevention and infectious diseases control. SATREPS is sponsored by the Japan International Cooperation Agency (JICA) and the Japan Science and Technology Agency (JST). It was launched in 2008. The JICA and JST are the organizations under the Ministry of Foreign Affairs of Japan, and the Ministry of Education, Culture, Sports, Science and Technology of Japan, respectively. Under the umbrella of the SATREPS, we were able to obtain funds for a project entitled “Magmatic fluid supply into Lakes Nyos and Monoun, and the mitigation of natural disasters through capacity building in Cameroon”. This started in 2011. The project was nicknamed “SATREPS-NyMo”. It was a 5-year project and continued until March 2016. The project was headed by Professors Takeshi Ohba (Tokai University, Japan) and Minoru Kusakabe (co-leader, University of Toyama, Japan). The counterpart organization in Cameroon was the Institute for Geological and Mining Research (IRGM) headed by Dr. Joseph V. Hell, under the Ministry of Scientific Research and Innovation (MINRESI). The goal of the project was to mitigate natural disasters in Cameroon through capacity building, specifically for issues related to the Lakes Nyos and Monoun gas disasters. To accomplish the goal, we planned the following sub-projects: (1) a CO2 discharge system beneath Lakes Nyos and Monoun; (2) the hydrological regime around the lakes; (3) the eruptive history of volcanoes along the Cameroon Volcanic Line (CVL); (4) the CO2 distribution in Lakes Nyos, Monoun and other lakes along the CVL; (5) the setup of an experimental system for removing CO 2rich deep water to prevent gas re-buildup in Lake Monoun; and (6) the continuation of geochemical Fig. 31. Schematic presentation of the deep water removal system. monitoring of Lakes Nyos and Monoun. During the project, scientific cooperation between the two countries was encouraged through the exchange of scientists. Capacity building included scholarships to train Cameroonian students and technicians in Japan, and the donation of scientific instruments to IRGM. The progress of the SATREPS-NyMo can be seen in the website “http://www.satreps.u-tokai.ac.jp”. The project went well in terms of scientific achievement. Many scientific papers were published, e.g., Issa et al. (2013, 2014a, 2014b), Asaah et al. (2014, 2015), Chako Tchamabé et al. (2013), Fouépé et al. (2013), Fantong et al. (2013, 2015), Tiodjio et al. (2014, 2015, 2016), Kamtchueng et al. (2014, 2015a, 2015b), Yoshida et al. (2016), Ohba et al. (2016), Kozono et al. (2016), and Saiki et al. (2016). As described in Section 3, a bottom water removal system was installed at Lake Monoun in December 2013 to stop re-buildup of CO2 (Yoshida et al., 2016). The system is shown in Figs. 31, 32. As the degassing pipes in Lake Monoun had lost their gas self-lift capability, one of the pipes was utilized to set up a solar power driven rotary pump, in order to reduce the total cost of the installation. The intake depth of the pipe is ~99 m, very close to the bottom (100 m). A small rotary water pump with an outer diameter of 74 mm was placed inside the pipe which had an internal diameter of 100 mm. Four small solar modules with a total output of 320 W were used as a power source. Although the system shown in Fig. 32 works only during the daytime, it is capable of pumping bottom water at an doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. 32 M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 Fig. 32. Photograph showing the solar power-driven deep water removal system installed at Lake Monoun. estimated rate of ~100 m3/day. Based on this rate and the current CO 2 concentration of deep water (~90 mmol/kg as of March 2014), the annual removal rate of CO2 is calculated to be 3.3 Mmol/year. Since this removal rate is less than half the natural recharge rate (8.2~8.4 Mmol/year, Kling et al., 2005; Kusakabe et al., 2008), it would be advisable to install 2 additional systems at Lake Monoun to equal the natural CO2 recharge, in order to reduce the risk of a limnic eruption in the future. The system is robust and can work for a long time without complicated maintenance and transportation of fuel, which is an important factor that any system should have in a remote area like Lakes Nyos and Monoun in Cameroon. 6. The Cameroon Volcanic Line 6-1. Eruption age of the Nyos maar and potential collapse of the natural dam Lakes Nyos and Monoun are maar crater lakes situated along the Cameroon Volcanic Line (CVL) (Fig. 1). The northern edge of Lake Nyos consists of a 45m-wide natural dam (Fig. 33) that holds surface lake water down to a depth of 40 m. The dam (Fig. 33) is made of pyroclastic materials deposited at the time of the volcanic eruption that formed the maar. The upper unit is moderately consolidated with visible cracks at the surface, whereas the lower unit is poorly consolidated and looks readily eroded as indicated by a concave structure beneath the upper unit (Lockwood et al., 1988). Erosion of the lower unit may be facilitated by seeping water. Lockwood and Rubin (1989) determined 14C ages of 2 pieces of charcoal found at the base of the lower unit to be ~400 and ~5100 years BP (before present). They took the age of 400 years to indicate the age of trees that were growing at the time of maar formation. The older age was discarded based on Fig. 33. Photograph showing the 45-m-wide natural dam at the northern edge of Lake Nyos. The area surrounded by a green curve is the head of the valley where pyroclastic materials are said to have been eroded away. an interpretation that the trees grew in magmatic CO2rich atmosphere at the center of the present maar where the eruption took place. Magmatic CO2 is characterized by “dead carbon” (no or very little 14C), and its incorporation in trees resulted in older ages. The pyroclastic rocks that form the dam once extended much farther to the northwest (~600 m), but the lake water overflowing the spillway has back-eroded these rocks along the stream bed, leaving only the 45-m-wide dam at the present time (Fig. 33). An average erosion rate calculated from these data is 1.5 m/year. At this rate, the 45-m-wide dam will be eroded away in 30 years, if the age of the dam is correct and the erosion proceeds at the mean constant rate. It is, however, more realistic to imagine that the dam collapse will take place in an irregular and catastrophic way. Figure 34 shows many joints at the surface of the moderately consolidated upper unit and the seepage of lake water through the poorly consolidated lower unit. The seepage of CO2-containing lake water may have chemically eroded the lower unit in the past, resulting in fall-out of the lower unit, as suggested by the existence of caves. There may be an associated breakage of the jointed upper unit. Thus, the erosion rate may vary irregularly with time, but it is still alarmingly high. On this basis, Lockwood et al. (1988) warned that the dam may eventually collapse releasing >50 million tons of water and inducing a catastrophic flood on downstream areas including part of Nigeria. This warning was seriously taken up by the Cameroonian authorities. They asked support from the United Nations Office for the Coordination of Humanitarian Affairs (OCHA) and the United Nations Environmental Program (UNEP) for a detailed survey of the dam. A team of experts from OCHA and UNEP concluded that a failure of the dam doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 33 Fig. 34. Cross-section of the natural dam at Lake Nyos. Some explanatory words were added on the cross-section originally drawn by Lockwood et al. (1988). Figure 3 of Bull. Volcanol., The potential for catastrophic dam failure at Lake Nyos maar, Cameroon, 50, 1988, 340–349, Lockwood, J. P., Costa, J. E., Tuttle, M. L., Nni, J. and Tebor, S. G., „ Springer-Verlag 1988 with permission of Springer. is highly likely “to occur within the next 5 years” based on their geotechnical survey (Joint UNEP/OCHA Environment Unit, 2005). They recommended reinforcement of the dam by cementing fractures and the unconsolidated part of the dam. As a result, the dam has been reinforced by engineering methods. The warning by Lockwood et al. (1988) also drove geochronological studies of the dam, since the age is directly related to the erosion rate and thus to the safety of the dam. The age of the dam has long been debated. The debate on the age is concisely summarized in Aka and Yokoyama (2013). Freeth and Rex (2000) proposed that the age of eruption of the Nyos maar was in excess of 100,000 years, based on K-Ar dates (Fig. 34) and evidence from aerial photographs taken in 1963– 1964 that showed no change in the width of the dam since that time. They concluded that the dam materials were eroding at a “geologically realistic rate” and that “there is no reason to suspect that the rate at which it is currently eroding away is, in itself, sufficient to pose an immediate threat”. However, the application of the K-Ar dating method to the basaltic rocks from the Nyos dam area was criticized by Lockwood and Rubin (1989), because the Nyos basalts contain fine shards of K-feldspars which were derived from basement monzonite (Fig. 34). The K-Ar dating of the rocks containing the shards gives much older ages than their true age due to the inclusion of K-feldspars with a high radiogenic Ar concentration. Aka et al. (2008) applied a U-series dating method to Lake Nyos maar basalts. The basic principles, assumptions and applications of the U-Th dating method are summarized in Chabaux and Allègre (1994). Aka et al. (2008) analyzed 12 samples collected from the Lake Nyos area, including 5 samples of the dam-forming surge deposit and 5 nearby lava flows. They used XRF and ICP-MS for the analysis of major and trace element compositions including (238U/ 232Th), (230Th/ 232 Th), ( 226Ra/230Th) and (238 U/230Th) ratios. The results of the Th-Ra disequilibria are reproduced in Fig. 35. The (230Th/232Th) ratios of 10 alkaline rock samples vary from 0.886 to 1.024, and the (238U/232Th) ratios vary from 0.716 to 0.880. Data for 26 samples from the Mt. Cameroon volcano, which has erupted during the last 100 years, are also included (Yokoyama et al., 2007). The Lake Nyos and Mt. Cameroon samples lie closely on a line marked as 238U/ 230Th = 0.82 with a few exceptions, significantly above the equiline which is 238U/ 230Th = 1.00. This feature indicates the presence of a 15 to 28% enrichment of 230Th over 238 U, suggesting strongly that the Lake Nyos maar formation is younger than ~375 ka which is 5 times the half-life of 230Th. If the time which has elapsed since the volcanic eruption is greater than 375 ka, then (230Th/ 238 U)A (activity ratio) becomes unity, or a secular equilibrium is established, and no dating can be made (equiline in Fig. 35a). Tholeiitic samples, D26 and D27 in Fig. 35a plot on the line 238U/ 230Th = 1.00, an indication that they are in the 238U-230Th radioactive equilibrium, giving their formation age older than 375 ka, with no more information about the age. It is important to note that the variation in the (230Th/232Th)A and (238U/ 232 Th)A ratios of the Mt. Cameroon samples (~0.99 and ~0.82, respectively), and the corresponding excess 230 Th over 238U (18–24%) were all within the range for Lake Nyos samples (Fig. 35a). Figure 35b is a doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. 34 M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 Fig. 35. (a) ( 230Th/ 232Th)-(238U/232Th) activity ratio diagram for Lake Nyos and Mt. Cameroon samples. Some Lake Nyos samples are enriched in 230Th compared to 238U by 15–28%. (b) (226Ra/230Th)-( 238U/230Th) activity ratio diagram for the samples showing 2–19% excess 226Ra over 230Th, suggesting a Th-Ra fractionation of <10 ka BP. Reproduced from figure 4 of J. Volcanol. Geotherm. Res. 176, Aka, F. T., Yokoyama, T., Kusakabe, M., Nakamura, E., Tanyileke, G., Ateba, B., Ngako, V., Nnange, J. and Hell, J., U-series dating of Lake Nyos maar basalts, Cameroon (West Africa): Implications for potential hazards on the Lake Nyos dam, 212–224, Copyright 2008, with permission from Elsevier. plot of (226Ra/230Th)A vs. (238U/ 230Th)A for the studied samples. They were compared to published data for MORB and OIB (inset). The (226Ra/230Th)A ratios for the alkaline rock samples range from 1.017 to 1.040 with a mean value of 1.028 ± 0.008. These Nyos data plot above the (226Ra/230Th) A =1 line (equilibrium), indicating an enrichment of 226Ra compared to 230Th that was acquired during partial melting of the mantle source, as is generally observed in oceanic basalts (Thomas et al., 1999). Similar to 238U-230Th systematics, the tholeiitic samples are in 226Ra-230Th equilibrium. It is highly contrasting that the Mt. Cameroon data have higher (226Ra/230Th)A ratios (1.09–1.21) than the Lake Nyos samples (1.01~1.04), although the two volcanoes are similar in their degree of 238U-230Th disequilibria (Aka et al., 2008). The initial 226Ra/230Th ratio has to be known to calculate the age of the dam using the excess 226Ra. Since there are no eruptions of a known age which have occurred in the Lake Nyos area, the assumption was made that the initial ratio was the same (1.15 ± 0.02) as that measured in Mt. Cameroon lavas that are erupting today (Yokoyama et al., 2007). Using this assumption, the 226Ra- 230Th age of Lake Nyos was calculated to be 8.75 ± 0.49 ka (Aka and Yokoyama, 2013) after a careful examination of the samples. Based on this age, they consider that a collapse of the Nyos dam from erosion alone is not as imminent and alarming as has been suggested. However, making the dam more stable is necessary to completely eliminate the potential flood hazard. Stabilization by grouting of the dam has been undertaken. doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 35 Fig. 36. Chemical composition of rocks from CVL volcanoes. (a) K 2O+Na 2O versus SiO 2, and (b) Mg number versus SiO2 plots. Reproduced from figure 2 of Asaah et al. (2014) which should be referred to for the abbreviations. 6-2. Origin of the Cameroon Volcanic Line The Cameroon Volcanic Line (CVL) is an alignment of Cenozoic volcanoes stretching for 1600 km from Annobon in the Gulf of Guinea to Biu Plateau in the continental part of central Africa (Fig. 1). It straddles both oceanic and continental lithosphere. The CVL can be grouped into 3 sectors, i.e., the oceanic sector to the southwest (Annobon, Saõ Tomé, and Principe), the ocean-continent boundary (Bioko, Etinde and Mt. Cameroon), and the continental sector (Manengouba, Bambouto, Oku, Ngaoundéré Plateau, Mandara Mountains and Biu Plateau) to the northeast. The volcanic islands in the oceanic sector are made up of rocks ranging from nephelinite, basanite and basalt to trachyte and phonolite (Halliday et al., 1988; Deruelle et al., 1991). The volcanoes in the ocean-continent boundary are located SW of Mt. Cameroon, and are made of mostly nephelinitic lavas for Etindé (Nkoumbou et al., 1995) and basalts and basanites for Bioko and Mt. Cameroon (Yokoyama et al., 2007; Asaah et al., 2014). Mt. Cameroon is the only active volcano in the CVL doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. 36 M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 Fig. 37. Trace element patterns for mafic rocks from the oceanic and continental sectors of the CVL. Reproduced from figure 4 of Asaah et al. (2014). The patterns are generally similar to each other and akin to OIB, suggesting an origin from a similar source. Reproduced from figure 5 of Asaah et al. (2014). with seven eruptions recorded in the last 100 years, i.e., 1909, 1922, 1954, 1959, 1982, 1999, and 2000 (Suh et al., 2003). It is a composite volcano made of alkaline basanitic and basaltic flows interbedded with small amounts of pyroclastic materials and numerous cinder cones (Suh et al., 2003; Yokoyama et al., 2007). The continental sector of the CVL includes Mount Bambouto and Mount Oku. They are Oligocene to Quaternary strato volcanoes with lava successions comprising a strongly bimodal basalt-trachyte-rhyolite suite (Marzoli et al., 2000, 2015; Kamgang et al., 2013). Mt. Manengouba is also in the continental sector and is a well-preserved stratovolcano whose summit hosts two concentric calderas with lakes. Lavas range from basalts to trachytes, quartz trachytes, and rare rhyolites (Pouclet et al., 2014). The Ngaounderé Plateau in the northeastern continental part of the CVL consists of alkaline basalts and basanites capped by trachytes and phonolitic flows. The Biu Plateau, which is located in the northern part of the Ngaounderé Plateau, consists of basaltic flows with a maximum thickness of 250 m. This plateau is composed of basanite to transitional basalts (Rankenburg et al., 2005). Since Lakes Nyos and Monoun are situated on the CVL, it may be informative to give a brief summary of the origin of the CVL to understand the characteristics of the Nyos and Monoun volcanoes. The origin of the CVL has long been a subject of controversy, and various hypotheses have been proposed. They are summarized by Aka et al. (2004) and more recently by Asaah et al. (2014), as follows: (1) Reactivation of preexisting tectonic structures in the Cenozoic associated with crustal melting (Gorini and Bryan, 1976; Moreau et al., 1987; Fairhead, 1988). (2) Membrane stresses generated by the movement of the African plate away from the equator (Freeth, 1978). (3) Displacement of the African plate (Fitton, 1980). (4) Hotspot trail (Morgan, 1983). (5) Hotline hypotheses (Meyers et al., 1998). (6) A plate-wide shallow mantle convection model (Burke, 2001). (7) Edge convection and lithospheric instability (Reusch et al., 2010). Of the above, Fitton’s classic hypothesis is still attractive in that the Benue Trough and the CVL are related to a common “Y”-shaped hot zone in the asthenosphere over which the African plate moved during the period of 110 to 70 Ma (Fitton, 1980). The “Y”-shaped hot zone was a rift zone that extended from a triple junction originally located at the Gulf of Guinea that was underlain by the St. Helena hotspot at the time of the opening of the south Atlantic. The CVL developed over this rift zone. In this sense, the magmatism in the CVL may have been similar to that in the currently active East African Rift Zone. Asaah et al. (2014) went for the “hotline” model which invokes multiple plumes doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 37 Pb/ 204Pb- 206Pb/ 204Pb diagram, and (b) 143Nd/ 144Nd-87Sr/ 86Sr diagram for the CVL lavas. (c) Positive and negative trends were seen in the 143Nd/ 144Nd-206Pb/ 204Pb relationship for the CVL lavas. Reproduced from figures 6, 7 and 9 of Asaah et al. (2014). EM1 is for Enriched Mantle type 1, EM2 for Enriched Mantle type 2, NHRL for Northern Hemisphere Reference Line, HIMU for high- m (=238U/204Pb ratio), FOZO for Focal Zone, MORB for Mid-Ocean Ridge Basalt, OIB for Ocean Island Basalt, and DMM for Depleted MORB Mantle. Fig. 38. (a) 207 originating from the same source in the upper mantle, each of which produced volcanoes independently, as the model appears to explain the diverse features of the CVL, i.e., geophysical, structural and geochemical evidence, including the absence of time-dependent volcanic activity. Magmatism of the CVL is characterized by melting in the garnet lherzolite stability fields (Marzoli et al., 2000; Yokoyama et al., 2007; Kamgang et al., 2013), although melting in the spinel lherzolite stability field doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. 38 M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 has been reported in the Ngaoundere Plateau (e.g., Lee et al., 1996; Nkouandou and Temdjim, 2011). In addition, mixing of both garnet and spinel melting fields has been reported in Mt. Cameroon (Tsafack et al., 2009). Both mafic and felsic rocks show chemical features consistent with a plume activity outlined by their ocean island basalt (OIB) characters, and their isotopic ratios (Mbassa et al., 2012). 6-3. Geochemistry of CVL magmas Asaah et al. (2014) made a comprehensive review of the geochemistry of CVL rocks. They compiled the existing geochemical data of the CVL rocks (580 samples) consisting of major and trace element compositions and radiogenic (Sr-Nd-Pb) isotope compositions. Figure 36 shows some chemical characteristics of the CVL rocks in terms of (a) K2O+Na2O versus SiO2, and (b) Mg number versus SiO2 plots. The SiO2 contents show a wide range of variation from 38% (oceanic CVL) to 79% (continental CVL) reflecting the diverse rock types. The Mg number (Mg#), defined as Mg# = MgO/(MgO+FeO)*100, is often used as an index of the level of evolution of volcanic rocks. It shows different trends from one volcanic center to another. The CVL rocks from the oceanic and continental sectors are dominantly alkali basalts and basanites. The Mg# of mafic samples ranges from 60~69 (least evolved basalts) to 40~49 (evolved rocks), indicating various fractional crystallization paths (Fig. 36b). Refer to Asaah et al. (2014) for further discussion. Abundance patterns of trace elements are often used to discuss magma genesis, since they provide geochemical and geological information through their unique chemical properties and sensitivity to processes to which major elements are insensitive. Primitivemantle normalized trace element patterns (Palme and O’Neill, 2003) for mafic rocks from the oceanic and continental sectors of the CVL are presented in Fig. 37. The patterns are generally similar to each other (except for the Mt. Etindé samples) and akin to OIB, suggesting an origin from a similar source. They show a marked enrichment of light rare earth elements (LREEs) and a strong fractionation of heavy rare earth elements (HREEs) relative to LREEs. The most striking features of Fig. 37 are: (1) the Mt. Etindé samples have high trace elemental abundances compared to the other CVL alkaline basalts; (2) relatively high positive anomalies of Nb, La and Nd; and (3) the occurrence of a K-trough. Nearly constant elemental ratios of incompatible elements in CVL rocks suggest that magma processes, such as zone refining melting, magma mixing, and extensive fractionation and replenishment, were not dominant processes during the generation of the CVL mafic lavas, because the above processes can efficiently fractionate incompatible elements. The peculiar features of Mt. Etindé may have resulted from source materials that are different from the other CVL lavas, as suggested by the Mg# versus SiO2 trend (Fig. 36b) and a strong high m (HIMU) character there. The radiogenic isotope (Sr-Nd-Pb) geochemistry of the CVL rocks is also summarized in Fig. 38, adapted from Asaah et al. (2014). The Sr-Nd-Pb isotopic compositions of the CVL basalts overlap those of OIB. In the 207Pb/204Pb vs. 206Pb/204Pb diagram (Fig. 38a), the data plot parallel to the Northern Hemisphere Reference Line (NHRL) of Hart (1984), and to the right of the 4.53 Ga geochron. However, some data from the Oku Volcanic Group (OVG) with low 206Pb/204Pb and 207 Pb/204Pb ratios overlap with the MORB end members (Atlantic, Indian, and Pacific MORBs). The 143Nd/ 144 Nd ratios and 87Sr/86Sr ratios of the mafic rocks show a limited range of variation (Fig. 38b). The 87Sr/86Sr ratios range from 0.70286 in a sample from the Biu Plateau to 0.70515 in a sample from Mt. Bambouto. The 143Nd/144Nd ratios vary from 0.51302 in a sample from the Biu Plateau to 0.52771 in a sample from Mt. Cameroon. Some lavas from the Biu Plateau and the oceanic CVL show relatively low 87Sr/ 86Sr and high 143 Nd/144Nd ratios, implying that they are more primitive than other continental volcanic rocks (Mt. Bambouto, Mt. Manengouba, and the OVG). Isotope data for the OVG show a wider spread than those of the other CVL volcanoes. This difference is conspicuous in the Pb isotopes. In Fig. 38b, a negative correlation is observed between 143Nd/144Nd and 87Sr/86Sr ratios and the correlation slope matches the mantle array of MORB-OIB samples. From these figures it is suggested that the CVL lavas formed by a dominant contribution of EM2 to the Depleted MORB Mantle (DMM). The 143Nd/144Nd versus 206Pb/ 204Pb plots (Fig. 38c) show positive and negative correlations with different slopes, where the role of EM2 becomes dominant over EM1. Mixing with various end members in different proportions may account for the complex isotopic characteristics of the CVL lavas. Based on trace element and isotope geochemistry, it has been suggested that these magmas derived from the sub-lithosphere without interaction with the overlying lithosphere (Fitton and Dunlop, 1985). A different view, however, was given by Halliday et al. (1990) that the continent/ocean boundary magmas (Bioko, Etindé and Mt. Cameroon) are characterized by 206Pb/ 204 Pb ratios that are higher (more radiogenic) than those of typical continental and oceanic sector magmas. This radiogenic feature has also been confirmed by the distribution of 3He/ 4He ratios of lavas and mineral separates from the CVL rocks showing a clear 3He/ 4He valley as already illustrated in Fig. 20 (Aka et al., 2004). Together with Sr, Nd and O isotopic variations, Halliday et al. (1988, 1990) suggested that the radiogenic nature of the 206Pb/ 204Pb ratios of rocks from the ocean-continent boundary reflects melt migration from the St. Helena fossil plume head that took place at 125 doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 39 Fig. 39. Schematic presentation of a model for source enrichment in high m elements following plume emplacement at 125 Ma beneath the ocean/continent boundary of CVL (St. Helena). Reprinted by permission from Macmillan Publishers Ltd: Nature 347, 523–528, Halliday, A. N., Davidson, J. P., Holden, P., DeWolf, C., Lee, D.-C. and Fitton, J. G., Trace-element fractionation in plumes and the origin of HIMU mantle beneath the Cameroon line, Copyright 1990. Ma, and that some of the CVL magmas derive from the upper metasomatized part of the fossil plume in the lithospheric mantle (Fig. 39). The degree of traceelement enrichment (U/Pb, or m in this case) varies as a function of the vertical thickness of the plume head through which the melts migrated. At the margins where the flattened plume head is thinnest, the source regions are dominated by more depleted mantle (Halliday et al., 1990). The radiogenic nature of 206Pb/ 204 Pb ratios and the 3He/4He valley observed at the ocean-continent boundary region can be explained by the magma genesis affected by the fossil plume head. 6-4. Volatiles in magma A fundamental question arises as to whether Lake Nyos magmas are enriched in CO2. Volatile contents in pre-eruptive magmas have been estimated by various techniques. One of the techniques is to analyze melt inclusions in phenocrysts, since melt is trapped in growing phenocrysts as melt inclusions in magma and is quenched to glass at the time of eruption. The volatiles, mainly H 2O and CO2, in the glass inclusions are determined by microanalytical techniques such as Fourier transform infra-red spectroscopy (FTIR), laser-Raman spectrometry, and secondary ion mass spectrometry (SIMS), etc. (Ihinger et al., 1994). Another approach is experimental petrology where mineral stabilities and assemblages are calibrated under different (but controlled) conditions, such as temperature, pressure, and water fugacity. Comparison of experimental products with natural phenocrystic assemblages allows us to constrain the pre-eruptive volatile contents (Johnson et al., 1994). Unfortunately for us, however, lavas from the Nyos volcano are mostly aphyric (Aka et al., 2008) and difficult to use for the analysis of pre-eruptive volatile contents by the aforementioned techniques. Instead, based on major and trace elements systematics, Aka (2015) proposed that the Nyos basalts formed by a small degree (1~2%) of partial melting of the primitive mantle to which amphibole and phlogopite had been added by carbonatitic fluids, and that decarbonation reactions of the carbonatitic metasomatism are responsible for producing the magmatic CO 2 . However, based on the geochemical data of the Nyos volcanic rocks, Asaah et al. (2015) suggest that CVL magmatism is predominantly of an asthenospheric source with little contribution from the subcontinental lithospheric mantle (SCLM). The lavas show evidence of enrichment by metasomatic fluids probably in the Mesozoic (e.g., Halliday et al., 1990; Aka, 2015; Asaah et al., 2015). The metasomatism affected the SCLM, inducing hydrous minerals like amphibole and phlogopite that are not stable in the asthenosphere. Asaah et al. (2015) suggest that the metasomatic fluids crystallized as small pockets or veins in the SCLM. An ultimate source of CO 2 in the Nyos magma may derive from the decarbonation of such crystallized metasomatic fluids. It is unlikely that the CVL magmas, including the Nyos magma, have abnormal CO2 in their mantle source. Lake Nyos and Lake Monoun volcanoes are located in the Oku and Bambouto volcanic centers, respectively, in the middle of CVL (Fig. 1). Lake Nyos is a maar lake created by a phreato-magmatic eruption. There are some other maar lakes near Lake Nyos, i.e., Oku, Elum, Nyi, Wum and Enep, but only Lake Nyos contains a large amount of dissolved CO2. According to Lockwood and Rubin (1989) who described the geology of the Nyos volcano, eruption sequences are sum- doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 40 Table 4. Concentration of volatiles in magma and expected gas composition from the magma. Concentration in magma Expected gas compostion H2O wt% CO2 ppm S ppm Cl ppm 0.12 0.20 1630 1320 690 1170 Melt inclusions from hotspot basalts Kilauea, ocean floor 0.46 Kilauea, summit 0.23 3100 800 Melt inclusions from subduction zone volcanoes Basalt 1.0 >1000 Andesite 3.0 >1000 Rhyolite 5.0 >1000 Glassy margin of MORB Eastern Pacific Ocean Atlantic Ocean H2O mmol/mol CO2 mmol/mol S HCl mmol/mol mmol/mol 50 60 526 621 292 167 170 203 11 9 1050 1300 æ 80 712 677 196 96 91 215 æ 12 1000 400 100 1000 3000 2000 871 933 971 36 13 8 49 7 1 44 47 20 Shinohara (2003) and Giggenbach (1996). marized as shown below. The formation of the Nyos maar is directly related to the ascent of alkali basalt magma. The first lava reached the surface in a relatively gentle, fire-fountaining fashion, depositing scoria and fluid bombs over a wide area around the present north end of Lake Nyos. This phase was followed by an explosive and violent eruption due to volatile expansion. The violence of the activity increased rapidly, however, and basalt is only included as shattered fragments in upper parts of the pyroclastic section. Neither the basalt flow nor the associated scoria at the base of the pyroclastic section were found to contain ultramafic xenoliths, suggesting that mantle rocks were only transported to the surface during the later more explosive phases of the eruption. The depth of the explosive activity may have gradually increased during the eruption, and the initial explosion crater gradually widened, which resulted in the formation of the maar crater, or the present Lake Nyos. It can be imagined that the magma subsided after the eruption, but the release of CO2-rich volatiles from the magma continues until today. Magma is generally generated by a partial melting of rocks in the lower crust or upper mantle. Mantle rocks, mainly comprised of peridotite, exist as a solid, because the geothermal gradient within the Earth is generally below the solidus of mantle rocks. It has been hypothesized that part of solid mantle, if heated locally, can ascend as a diapir and cross the solidus where partial melting starts to take place. Volatile materials such as H2O and CO2, if they coexist with the rocks, reduce the solidus temperature and facilitate a partial melting of the rocks, or magma genesis. Thus, the coexistence of volatiles is important for partial melting. Metasomatic fluids may have affected the primitive mantle beneath Lake Nyos and the fluids produced by the decarbonation of metasomatized mantle facilitated partial melting (Aka, 2015). Once a melt is formed, it rises through the mantle due to its lower density (higher buoyancy) and approaches the surface of the Earth to form magma. The melt may remain as a magma reservoir in the shallow part of the crust. When the magma further ascends, crystallization in the magma begins because of the reduction of temperature and pressure. Magma contains various volatile materials, such as H2O, CO2, S, Cl, etc. Since the volatile materials will not all be incorporated into crystals (or minerals), they tend to be concentrated as fluids in the magma as it rises and cools. A volcanic eruption is often facilitated by magma ascent driven by a lowered density due to the accumulation and expansion of bubbles of the volatiles in the magma. The chemical composition and concentration of magmatic volatiles have been estimated through the analysis of high temperature volcanic gases, chilled glassy margins of lava that has extruded onto the bottom of the deep ocean, and glass inclusions in phenocrysts of volcanic rocks. Volatiles in magma are almost completely discharged into the atmosphere at the time of volcanic eruption. For this reason, the chemical analysis of high-temperature volcanic gases, if collected and analyzed properly, can give the volatile composition (not concentration) in magma. Table 4 shows the concentration of H2O, CO2, S and Cl in some types of magma, and the composition of volcanic gases that is expected from the degassing of each type of magma (Shinohara, 2003). The concentration of the magmatic volatiles in magma is highly variable depending on its type. Water concentration in Mid-Oceanic Ridge Basalt (MORB) is low (0.1~0.5 wt%), whereas that of subduction zone magma is more than an order of magnitude higher (1~5 wt%). The concentration of CO2 in doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 41 Fig. 40. Solubilities of H2O and CO 2 in silicate melts (Holloway and Blank, 1994; Shinohara, 2003). MORB magma is 1000~3000 ppm, which is generally higher than that of subduction zone magma. The calculated composition of gases exsolved from magma is also shown in Table 4. Such calculated gas compositions are in general agreement with the observed gas compositions (not shown, but can be found in Allard, 1983; Gerlach, 1983; Shinohara, 2003). In gases from hotspot basaltic volcanoes such as Kilauea (Hawaii), the water content is lower than that from subduction volcanoes, whereas the CO 2 content is significantly higher in hotspot and mid-oceanic volcanoes, reflecting its higher concentration and low solubility in basaltic melts. Solubilities of H2O and CO2 in silicate melts have been experimentally determined as shown in Fig. 40 (Holloway and Blank, 1994, and references therein). The solubility depends on the temperature, pressure and the chemistry of melts. It increases as the partial pressure of the volatile species in question increases, and decreases as the temperature of the melt increases. Generally speaking, water is approximately an order of magnitude more soluble than CO2. Water dissolves slightly more in silicic melts than in basaltic melts, whereas CO 2 dissolves more in basaltic than in silicic melts (Fig. 40). Figure 41 illustrates the solubility of CO2 and H2O in basaltic melts at 1200∞C as a function of the total pressure of the volatiles. The non-linear relationship of this binary system in the melts comes from the non-ideal mixing properties of these species (Holloway and Blank, 1994). Using Fig. 41, it can be envisaged how the volatile composition in the melt changes as the decompression proceeds. For example, at point A of Fig. 41, where CO 2 = 540 ppm and H2O = 1.6 wt%, the melt is saturated with the coexisting fluid of which the mole fraction of H2O equals 0.2 and that of CO 2 is 0.8. This implies that the fluid coexisting with the basaltic melt is extremely rich in CO2. As the magma ascends, or the confining pressure is reduced, the fluid exsolves, or degassing takes place. If degassing proceeds in a closed system, the fluid com- Fig. 41. Solubilities of CO2 and H 2O in basaltic melts at 1200∞C as a function of the total pressure of the volatiles (Holloway and Blank, 1994; Shinohara, 2003). position in the melt will follow the thin dotted line depending on the co-existing H2O concentration as shown in Fig. 41. If degassing takes place in an open system, the melt composition may follow a different path, as indicated by the thick long dashed line, since the CO2-rich fluid leaves the magma when the system becomes open due to the low CO2 solubility in the melts, making the remaining magma progressively CO2-poor, while the H2O concentration decreases only a little. As long as the magma keeps open-system degassing, CO2-rich fluid is continuously released from the magma. This solubility-controlled behavior of CO2 in basaltic magma may explain a CO2-rich nature of fluids separated from the magma. The ultimate source of CO 2 in the Nyos magma may derive from the decarbonation of crystallized metasomatic fluids in the doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. 42 M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 subcontinental lithosphere (Aka, 2015; Asaah et al., 2015). The permanent supply of such CO2 is likely to be responsible for the high concentration of CO2 gas in the fluids feeding into Lakes Nyos and Monoun. 7. Other CO2-rich volcanic lakes in the world Of 714 volcanoes in the world, 86 volcanoes host lakes (Pasternak and Varekamp, 1997). Information on the volcanic lakes is now available from the VOLADA (2013) database (https://vhub.org/resources/2822). Although Lakes Monoun and Nyos in Cameroon became notoriously famous because of the gas disasters in the mid-1980s, other CO2-rich volcanic lakes exist in the world, e.g., Lake Kivu (Democratic Republic of the Congo and Rwanda, see below for references), Laacher See (Germany) (Aeschbach-Hertig et al., 1996), Lake Van (Anatolia in eastern Turkey) (Kipfer et al., 1994), Lago Albano and the two Monticchio lakes (Italy) (Anzidei et al., 2008; Caracausi et al., 2009), Laguna Hule and Rio Cuarto (Costa Rica) (Alvarado et al., 2011), and Lac Pavin (France) (Aeschbach-Hertig et al., 1999). Of these lakes, Lake Kivu has been known to contain a high concentration of CO2 and CH4 in its deep water since well before the Lake Nyos event (e.g., Deuser et al., 1973; Tietze et al., 1980). Carbon dioxide dissolved in the lake is basically of magmatic origin, a situation similar to that in Lakes Nyos and Monoun in Cameroon, although the magmatic CO2 is mixed with a variable proportion of biogenic CO2. The lake is located along the East African Rift on the border between the Democratic Republic of the Congo (DRC) and Rwanda. The lake area is tectonically and volcanically active as part of the East African Rift System. Because of the high gas concentrations in the lake and the large population around it, Lake Kivu has a potential risk of a gas disaster caused by a limnic eruption which may be triggered by a possible volcanic eruption at the lake bottom (Schmid et al., 2005) or a plunge of lava flows from Nyiragongo, the nearest active volcano (only 20 km NE to the lake). Indeed, the 2002 eruption of the volcano generated lava from flank fissures flowed into the city of Goma, the provincial capital, resulting in destruction of local structures and the evacuation of local people, and these lava flows eventually ran into the lake. Fortunately, no limnic eruption was induced at that time (Tedesco et al., 2007). Detailed gas and water chemistry of Lake Kivu and the surrounding region has been published by several authors (Tietze et al., 1980; Tassi et al., 2009; Schmid et al., 2005). The lake has 5 basins, each of which is characterized by a different chemistry, CO 2 profile, and biology. The main basin (>250 m) contains the highest CO 2 concentration with a horizontal heterogeneity. Although the highest CO2 concentration in the main basin is far from saturation at any depth, the CO2 con- centration at Kabuno Bay (a small basin on the northwestern end of Lake Kivu) is relatively close to saturation. Since Kabuno Bay is shallower than the main basin and is characterized by the highest input of CO2rich magmatic fluid, the bay is considered to be potentially most hazardous in terms of the possibility of limnic eruption. Continuous monitoring is recommended (Tassi et al., 2009). The concentration of dissolved CH4 is highest (~17 mmol/L), approx. 12% of dissolved CO2 at the bottom of the main basin. The gas is produced by the bacterial reduction of CO2 and acetate fermentation (Schoell et al., 1988). It is important to note that microbial activity contributes to the gas chemistry in deep, stratified and anaerobic lakes, as recently found also at Lakes Nyos and Monoun (Tiodjio et al., 2014, 2016). Carbon isotopic ratios (d13C) of CO2 dissolved in the main basin of the Lake Kivu range from –7 to –6‰ (relative to VPDB), suggesting also a large contribution from mantle-originating CO2. Those at Kabuno Bay (–11 ~ –13‰), however, are significantly lower than the values for the main basin, probably reflecting the interaction of magmatic fluids with organic-rich sedimentary materials that underlie volcanic rocks derived from nearby Nyamulagira and Nyiragongo volcanoes (Tassi et al., 2009). The 3He/4He ratio of Kabuno Bay water is 5.5 Ratm, indicating a large contribution of a magmatic component regardless of the low d 13C values, whereas the ratio ranges from 2.1–2.6 Ratm in the main Kivu basin water. Fumarolic gases collected at the summit crater of the Nyiragongo volcano may best represent the 13C/12C and 3He/4He ratios of magmatic end-members in the fluids that are supplied to Lake Kivu and its surroundings (Tedesco et al., 2010). These authors observed typical mantle values of d 13C = –3.5 ~ –4‰ and 3He/4He ratios up to 8.7 R atm for the fumarolic gases. The influence of this magmatic signature becomes smaller, and the crustal components increase, as we move southward (toward Lake Kivu). The C/ 3 He ratio of ~30 ¥ 10 10 was observed for summit fumarolic gases. This high value probably reflects the high CO2 solubility in the Nyiragongo magma which is foiditic (alkaline), different from typical MORB magmas. High C/3He ratios up to 36 ¥ 1010 were measured for the main basin water of Lake Kivu. Although these ratios are close to the Nyiragongo magmatic value, it is more likely that the addition of CO2 in local groundwater that has interacted with organic materials enhanced the lake’s C/3He ratio, as suggested by d13C values. These observations show that magmatic fluids interact with surrounding materials in varying degrees, and that the gas geochemistry of this area is controlled by the local tectonic-geologic settings (Tedesco et al., 2010). Lake Mashu is a small, dimictic (mixing twice a year) caldera lake in Hokkaido, Japan, with a surface area of 19 km2 and a maximum depth of 211 m. A hot spring doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 has been identified at the bottom of the lake. The chemical characteristics of the lake were given by Nojiri et al. (1990). Based on noble gas data of the lake water collected at various time, points and depths, Igarashi et al. (1992) estimated a 3He/ 4He ratio of 6.7 Ratm for helium supplied from the lake bottom through the hot spring, suggesting the addition of mantle helium to the lake from the underlying magma. The accumulation of mantle helium between two overturns (spring and autumn) was estimated to be 9.2 ¥ 107 atoms cm–2 s–1 (4He) and 8.7 ¥ 102 atoms cm–2 s–1 ( 3He) using the helium profiles and a one-dimensional diffusion model. Since the CO2 supply rate was estimated to be 5.3 ¥ 10 8 mol/y (Nojiri et al., 1993), a high C/ 3He ratio of 18 ¥ 1010 can be calculated for the supply fluid. The C/3He ratio, similar to that estimated for the Alban Hills volcanic district (see below), is two orders of magnitude greater than the MORB value. Igarashi et al. (1992) attributed this high value to the enrichment of CO2 in the source magma beneath Lake Mashu. Although the CO2 supply rate of 5.3 ¥ 108 mol/y is greater than that for Lake Nyos (1.2 ¥ 108 mol/y, Kusakabe et al., 2008) by a factor of ca. 4, the dimictic nature of the lake does not allow an excessive accumulation of CO2 in it, which is fortunate from the limnic eruption perspective. Laacher See is also a 53-m-deep holomictic (complete vertical mixing once a year) maar lake in the East Eifel volcanic district in Germany, where the discharge of CO2 gas from the lake has been observed for years. Helium and neon isotopes dissolved in the lake were measured twice (spring and early autumn) in 1991 by Aeschbach-Hertig et al. (1996) with the aim of estimating the helium flux from the lake bottom, since gases supplied from the bottom were considered to accumulate in the lake during summer stratification. Both the He concentration and 3He/4He ratios increased with depth, and the rate of increase was more clearly observed in early autumn. The 3He/4He ratio of the incoming He was estimated to be 5.4 Ratm, suggesting a large contribution of magmatic He with a minor crustral contribution. Using the amount of He stored during summer stratification and a one-dimensional vertical mixing model, the 4He flux into the lake was estimated to be 10 ¥ 108 atoms/cm2 s–1 with a 3He/4He ratio of 5.3 Ratm. Since gas samples from the lake were >99% CO 2, a C/3He ratio of 8.6 ¥ 109 was calculated. Combining the 3He flux of 7.4 ¥ 103 atoms cm–2 s –1, a CO 2 flux into Laacher See was estimated to be 3.3 mmol cm –2 y–1 (Aeschbach-Hertig et al., 1996). This is equivalent to an annual release of 1.1 ¥ 108 mol CO2 to the atmosphere. Even if this value represents the annual recharge of CO2 to the lake, the holomictic nature does not allow the accumulation of CO2 as was the case in Lakes Nyos and Monoun. Lake Van in Anatolia, eastern Turkey, was formed during the Pleistocene in a tectonic depression with its 43 outlet blocked by lava flows from the nearby Nemrut volcano. Lake Nemrut is one of the caldera lakes near Lake Van. The injection of He, derived from depleted mantle with 3He/ 4 He ratio of 7.4 R atm, into Lakes Nemrut and Van was documented by Kipfer et al. (1994). It is likely that CO2 is also supplied to the lakes, but unfortunately there is no mention of CO2 in the lakes in Kipfer et al. (1994). Alban Hills in the volcanic area near Rome, Italy, has been characterized by high emissions of CO2 from a pressurized CO2-rich aquifer, and small-scale gas outbursts from the aquifer have been recorded (Carapezza and Tarchini, 2007). Carbon isotopic ratios were reported to be in a limited range around +1.3‰ (relative to VPDB), which suggests the contribution of decomposed marine carbonates as the source of CO2. The 3He/ 4He ratio of He in the associated gas was 1.9 Ratm, very low compared to MORB and subduction volcanic gas values, but still suggestive of a magmatic affiliation. The C/3He ratio of gases collected from a nearby well is 2.3 ¥ 1011, 2 orders of magnitude greater than typical MORB values. This value is consistent with a high contribution of CO2 that was most likely derived from the thermal decarbonation of limestone involved in magma genesis at the Alban Hills volcanic district. Historical evidence has shown that Lake Albano, a 160-m-deep crater lake located in the center of the district, experienced lahars associated with water overflow (Carapezza and Tarchini, 2007). The present water and gas chemistry of the lake indicates that dissolved CO2 concentration increases with depth in anoxic hypolimnion (>80 m). However, the total gas pressure calculated from the CO2 concentration is far below the hydrostatic pressure at all depths, suggesting that a gas hazard at the lake is unlikely, unless CO2 from the pressurized aquifer is suddenly injected into the lake (Carapezza et al., 2008). The Monticchio crater lakes in Southern Italy are also receiving passive magmatic CO2, and the potential risk of a Nyos-type gas hazard has been described (Caracausi et al., 2009). 8. Concluding remarks This review mainly summarizes the author’s achievements in work and related matters on the Lakes Nyos and Monoun gas disasters that took place in the mid1980s in Cameroon. At that time, nobody knew that lakes could accumulate so much CO2 gas and then suddenly release it to induce such disasters. The Lake Nyos and Monoun events had a strong impact on scientists working on gas emissions from the interior of the Earth. This impact especially boosted volcanic lake studies. Soon after the 1986 gas burst at Lake Nyos, scientists working on the initial phase of their research created a small informal group “The International Working Group on Crater Lakes (IWGCL)” to exchange scientific information about the Lake Nyos gas disaster, to doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. 44 M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 coordinate follow-up field trips planned by those who were interested in the subject, and to organize scientific meetings as a forum for further discussions. The scope of IWGCL was later expanded to include not only studies of gassy lakes in Cameroon but also those of other volcanic lakes in general. The new objectives were to obtain information on the activity and degassing state of shallow magmatic bodies so that forecasting volcanic eruptions and the mitigation of volcanic lake-related hazards could be achieved. Expansion of the scope of IWGCL naturally meant a greater number of scientists, and resulted in acquiring a formal IAVCEI status as the Commission on Volcanic Lakes (CVL) in 1993. I was satisfied by these organizational developments as the leader of IWGCL and CVL in those early days. The CVL has organized scientific meetings every 2–3 years, reports of which can be found in the website “http://www.ulb.ac.be/sciences/ cvl/”. On a personal note, and as a scientist who has worked on Lakes Nyos- and Monoun-related disaster reduction issues for close to 30 years, I was particularly happy to know that the CVL-9 meeting took place in Yaoundé, Cameroon, in March 2016, to commemorate the 30th anniversary of the Lake Nyos gas disaster. During my career, I have acquired experiences of working in national and international scientific communities, and, consequently, have made friendships with many wonderful scientists worldwide. Such experiences have led me to obtain research funding that has made it possible for me to continue to work in Cameroon for ~30 years. As described in Section 5 of this article, a typical example was the success in getting support from JICA and JST for the SATREPSNyMo project. The resolution of solving various problems associated with the Lakes Nyos and Monoun gas disasters, such as the continuation of scientific monitoring of the lakes, the monitoring of the reinforced natural dam at Lake Nyos, the rehabilitation and setting up of an infrastructure for the displaced people, etc., are obviously domestic issues for which the Cameroonian Government and scientists should, in principle, take responsibility. But the reality is different; the economic insufficiency of Cameroon has hindered the principle. The main goal of the SATREPSNyMo project is to mitigate natural disasters in Cameroon through capacity building, specifically for issues related to the Lakes Nyos and Monoun gas disasters. The risks of the recurrence of limnic eruptions can be defused if proper and timely actions are taken. The SATREPS-NyMo capacity building included the donation of some analytical instruments necessary to help Cameroonian scientists achieve the project’s goals. Also included was the training of young Cameroonian scientists and technicians in Japan, so that, after they get back home, they can play an important role in the field of mitigation of natural disasters. Unfortunately, the gas content at Lake Monoun has recently been found to be increasing due to the continuing gas supply from the underlying magma, the duration of which is much longer than the span of human life. It is almost certain that the same situation will occur at Lake Nyos within several years when the gas self-lift capability is lost. Now is the turn for Cameroonian scientists and technicians to work toward defusing the new risks of the increasing gas content in the lakes, for they have acquired the needed knowledge and techniques. I hope the safety of the lakes is secured and that the surrounding populations can return to their ancestral roots and go about their daily lives without the fear of further gas disasters. Acknowledgments This article is a review of scientific achievements concerning the Lakes and Monoun Nyos gas disasters and related subjects. The review could not have been made without the cooperation of many colleagues and friends who worked together with me in the field. I express my sincere thanks to: Y. Yoshida, T. Ohba, K. Nagao, G. Tanyileke, F. T. Aka, Issa, Y. W. Fantong, J. V. Hell, G. W. Kling, W. C. Evans, D. Rouwet, and many others, who worked together in the field and have provided me with important scientific information. Special thanks go to T. Ohba who kindly supplied recent data (unpublished) on the CO2 concentrations in the lakes used in Fig. 15. Fieldwork since in the period 1986–2006 was mostly supported by the Grant-in-Aid for Scientific Research from JSPS (Japan Society of Promotion of Science). Recent fieldwork (2011–2015) has been supported by the SATREPS-NyMo project. Logistic support from IRGM and its technicians is appreciated. The Embassy of Japan and the JICA office in Yaoundé are acknowledged for their help while I was in Cameroon. K. Oshida of TerraPub is acknowledged for giving me the chance to write this review. I also thank Y. Matsuhisa who made constructive comments on an early version of the manuscript. W. C. Evans, D. Rouwet and F. Aka are also thanked for their comments that helped improve the manuscript. The English of the final version was improved by D. Larner who kindly checked the manuscript in a very careful manner and suggested the corrections. References Aeschbach-Hertig, W., Kipfer, R., Hofer, M., Imboden, D. M., Wieler, R. and Signer, P. (1996) Quantification of gas fluxes from the subcontinental mantle: The example of Laacher See, a maar lake in Germany. Geochim. Cosmochim. Acta 60, 31–41. Aeschbach-Hertig, W., Hofer, M., Kipfer, R., Imboden, D. M. and Wieler, R. (1999) Accumulation of mantle gases in a permanently stratified volcanic lake (Lac Pavin, France). Geochim. Cosmochim. Acta 63, 3357–3372. Aka, F. T. (2000) Noble gas systematics and K-Ar chronology: Implications for the Cameroon Volcanic Line, West Africa. Ph.D. thesis, Okayama University, Japan. Aka, F. T. (2015) Depth of melt segregation below the Nyos maar-diatreme volcano (Cameroon, West Africa): Majortrace element evidence and their bearing on the origin of CO 2 in Lake Nyos. Volcanic Lakes (Rouwet, D., doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 Christenson, B., Tassi, F. and Vandemeulebrouck, J., eds.), Springer-Heidelberg. Aka, F. T. and Yokoyama, T. (2013) Current status of the debate about the age of Lake Nyos dam (Cameroon) and its bearing on potential flood hazards. Nat. Hazards 65, 875–885, doi:10.1007/s11069-012-0401-4. Aka, F. T., Nagao, K., Kusakabe, M., Sumino, H., Tanyileke, G., Ateba, B. and Hell, J. (2004) Symmetrical helium isotope distribution on the Cameroon Volcanic Line, West Africa. Chem. Geol. 203, 205–223. Aka, F. T., Yokoyama, T., Kusakabe, M., Nakamura, E., Tanyileke, G., Ateba, B., Ngako, V., Nnange, J. and Hell, J. (2008) U-series dating of Lake Nyos maar basalts, Cameroon (West Africa): Implications for potential hazards on the Lake Nyos dam. J. Volcanol. Geotherm. Res. 176, 212–224. Allard, P. (1983) The origin of hydrogen, carbon, sulfur, nitrogen and rare gases in volcanic exhalations: Evidence from isotopic geochemistry. Forecasting Volcanic Events (Tazieff, H. and Sabrrorux, J.-C., eds.), Chapt. 25, 337– 386, Developments in Volcanology 1, Elsevier, Amsterdam. Alvarado, G. E., Soto, G. J., Salani, F. M., Ruiz, P. and Hurtado de Mendoza, L. (2011) The formation and evolution of Hule and Rio Cuarto maars, Costa Rica. J. Volcanol. Geotherm. Res. 201, 342–356. Anzidei, M., Carapezza, M. L., Esposito, A., Giordano, G., Lelli, M. and Tarchini, L. (2008) The Albano Maar Lake high resolution bathymetry and dissolved CO 2 budget (Colli Albani volcano, Italy): Constrains to hazard evaluation. J. Volcanol. Geotherm. Res. 171, 258–268. Aramaki, S., Ohsumi, T., Kanari, S., Kusakabe, M. and Hirabayashi, J. (1987), Letha gas burst from Lake Nyos, Cameroon, August 1986. Kazan (Bulletin of the Volcanological Society of Japan) 32, 17–24 (in Japanese). Asaah, A. N. E., Yokoyama, T., Aka, F. T., Usui, T., Wirmvem, M. J., Tchamabe, B. C., Ohba, T., Tanyileke, G. and Hell. J. V. (2014) A comparative review of petrogenetic processes beneath the Cameroon Volcanic Line. Geoscience Frontiers (http://dx.doi.org/10.1016/ j.gsf.2014.04.012). Asaah, A. N. E., Yokoyama, T., Aka, F. T., Usui, T., Kuritani, T., Wirmvem, M. J., Iwamori, H., Fozing, E. M., Tamen, J., Mofor, G. J., Ohba, T., Tanyileke, G. and Hell, J. V. (2015) Geochemistry of lavas from maar-bearing volcanoes in the Oku Volcanic Group of the Cameroon Volcanic Line. Chem. Geol. 406, 55–69. Ballentine, C. J., Marty, B., Sherwood Lollar, B. and Cassidy, M. (2005) Neon isotopes constrain convection and volatile origin in the Earth’s mantle. Nature 433, 33–38. Barberi, F., Chelini, W., Marinelli, G. and Martini, M. (1989) The gas cloud of Lake Nyos (Cameroon, 1986). J. Volcanol. Geotherm. Res. 39, 125–134. Barfod, D. N., Ballentine, C. J., Halliday, A. N. and Fitton, J. G. (1999) Noble gases in the Cameroon Line and the He, Ne, and Ar isotopic composition of high-A (HIMU) mantle. J. Geophys. Res. 104, 29509–29527. Baxter, P. J., Kapila, M. and Mfonfu, D. (1989) Lake Nyos disaster, Cameroon, 1986: the medical effects of large scale emission of carbon dioxide? British Med. Jour. 298, 1437– 1441. Burke, K. (2001) Origin of the Cameroon Line of Volcano- 45 capped swells. J. Geol. 109, 349–362. Caracausi, A., Nuccio, P. M., Favara, R., Nicolosi, M. and Paternoster, M. (2009) Gas hazard assessment at the Monticchio crater lakes of Mt. Vulture, a volcano in Southern Italy. Terra Nova 21, 83–87, doi:10.1111/j.13653121.2008.00858.x. Carapezza, M. L. and Tarchini, L. (2007) Accidental gas emission from shallow pressurized aquifers at Alban Hills volcano (Rome, Italy): Geochemical evidence of magmatic degassing? J. Volcanol. Geotherm. Res. 165, 5–16. Carapezza, M. L., Lelli, M. and Tarchini, L. (2008) Geochemistry of the Albano and Nemi crater lakes in the volcanic district of Alban Hills (Rome, Italy). J. Volcanol. Geotherm. Res. 178, 297–304. Chabaux, F. and Allègre, C. J. (1994) 238 U-230Th- 226Ra disequilibria in volcanics: a new insight into melting constraints. Earth Planet. Sci. Lett. 126, 61–74. Chako Tchamabé, B., Youmen, D., Owona, S., Ohba, T., Németh, K., Ngapna, M. N., Asaah, A. N. E., Aka, F. T., Tanyileke, G. and Hell, J. V. (2013) Eruptive history of the Barombi Mbo Maar, Cameroon Volcanic Line, Central Africa: Constraints from volcanic facies analysis. Cent. Eur. J. Geosci. 5(4), 480–496, doi:10.2478/s13533-0120147-2. Chau, H. F., Kwok, P. K. and Mak, L. (1996) A model of gas buildup and release in crater lakes. J. Geophys. Res. 101(B12), 28,253–28,263. Chevrier, R. M. (1990) Lake Nyos: phenomenology of the explosive event of December 30, 1986. J. Volcanol. Geotherm. Res. 42, 387–390. Conway, E. J. (1958) Micro Diffusion Analysis and Volumetric Error. Macmillan, New York. Costa, A. and Chiodini, G. (2015) Modelling air dispersion of CO 2 from limnic eruptions. Volcanic Lakes (Rouwet, D., Christenson, B., Tassi, F. and Vandemeulebrouck, J., eds.), 451–465, Springer-Verlag, Berlin, Heidelberg, doi:10.1007/978-3-642-36833-2_19. Craig, H. (1961) Isotopic variations in meteoric water. Science 133, 1702–1703. Deruelle, B., Moreau, C., Nkoubou, C., Kambou, R., Lissom, J., Njonfang, E., Ghogomu, R. T. and Nono, A. (1991) The Cameroon line: a review. Magmatism in Extensional Structural Settings: The Phanerozoic African Plate (Kampunzu, A. B. and Lubala, R. T., eds.), 275–327, Springer, Berlin. Deuser, W. G., Degens, E. T., Harwey, G. R. and Rubin, M. (1973) Methane in Lake Kivu: New data bearing on its origin. Science 181, 51–53. Duan, Z. and Sun, R. (2003) An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 bar. Chem. Geol. 193, 257–271. Evans, W. C., Kling, G. W., Tuttle, M. L., Tanyileke, G. and White, L. D. (1993) Gas buildup in Lake Nyos, Cameroon: The recharge process and its consequences. Appl. Geochem. 8, 207–221. Evans, W. C., White, L. D., Tuttle, M. L., Kling, G. W., Tanyileke, G. and Michel, R. L. (1994) Six years of change at Lake Nyos, Cameroon, yield clues to the past and cautions for the future. Geochem. J. 28, 139–162. Fairhead, J. D. (1988) Mesozoic plate tectonic reconstructions of central South Atlantic Ocean: the role of West doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. 46 M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 and Central Rift system. Tectonophysics 155, 181–191. Faivre Pierret, R. X., Berne, P., Roussel, C. and Le Guern, F. (1992) The Lake Nyos disaster: model calculations for the flow of carbon dioxide. J. Volcanol. Geotherm. Res. 51, 161–170. Fantong, W. Y., Fouepe, A. T., Issa, Djomou, S. L. B., Banseka, H. S., Anazawa, K., Adelana, S. M. A., Mendjo, J. W., Aka, F. T., Ohba, T., Hell, J. V. and Nkeng, G. E. (2013) Temporal pollution by nitrate (NO3) and discharge of springs in shallow crystalline aquifers: Case of Akok Ndoue catchment, Yaounde (Cameroon). Afr. J. Environ. Sci. Technol. 7(50), 175–191. Fantong, W. Y., Kamtchueng, B. T., Yamaguchi, K., Ueda, A., Issa, Ntchantcho, R., Mengnjo, M. J., Kusakabe, M., Ohba, T., Zhang, J., Aka, F. T., Tanyileke, G. and Hell, J. V. (2015) Characteristics of chemical weathering and water-rock interaction in Lake Nyos dam (Cameroon): Implications for vulnerability to failure and re-enforcement. J. Afr. Earth Sci. 101, 42–55. Farrar, C. D., Sorey, M. L., Evans, W. C., Howle, J. F., Kerr, B. D., Kennedy, B. M., King, C.-Y. and Southon, J. R. (1995) Forest-killing diffuse CO 2 emission at Mammoth Mountain as a sign of magmatic unrest. Nature 376, 675– 678. Fitton, J. D. (1980) The Benue Trough and the Cameroon line—A migrating rift system in West Africa. Earth Planet. Sci. Lett. 51, 132–138. Fitton, J. G. and Dunlop, H. M. (1985) The Cameroon Line, West-Africa, and its bearing on the origin of Oceanic and Continental alkali basalt. Earth Planet. Sci. Lett. 72, 23– 38. Fouépé, T. A., Kuitcha, D., Fantong, W. Y., Ewodo, M. G., Haris, H. K., Issa and Ohba, T. (2013) Assessing groundwater nitrate pollution in Yaoundé, Cameroon: Modelling approach. World App. Sci. J. 23(3), 333–344, doi:10.5829/ idosi.wasj.2013.23.03.321. Freeth, S. J. (1978) A model for tectonic activity in West Africa and the Gulf of Guinea during the last 90 m.y. based on membrane tectonics. Geol. Rundsch 67, 675–688. Freeth, S. J. (1990) The anecdotal evidence, did it help or hinder investigation of the Lake Nyos gas disaster? J. Volcanol. Geotherm. Res. 42, 373–380. Freeth, S. J. (1994) Lake Nyos: can another disaster be avoided? Geochem. J. 28, 163–172. Freeth, S. J. and Kay, R. L. F. (1987) The Lake Nyos gas disaster. Nature 325, 104–105. Freeth, S. J. and Rex, D. C. (2000) Constraints on the age of Lake Nyos, Cameroon. J. Volcanol. Geotherm. Res. 97, 261–269. Freeth, S. J., Kling, G. W., Kusakabe, M., Maley, J., Tchoua and Tietze, K. (1990) Conclusions from Lake Nyos disaster. Nature 348, 201. Gerlach, T. M. (1983) Intrinsic chemical variations in high temperature volcanic gases from basaltic lavas. Forecasting Volcanic Events (Tazieff, H. and Sabroux, J. C., eds.), Chapt. 24, 323–336, Elsevier, Amsterdam. Gerlach, T. M. (2011) Volcanic versus anthropogenic carbon dioxide. EOS 92(24), 14. Giggenbach, W. F. (1990) Water and gas chemistry of Lake Nyos and its bearing on the eruptive process. J. Volcanol. Geotherm. Res. 42, 337–362. Giggenbach, W. F. (1996) Chemical composition of volcanic gases. Monitoring and Mitigation of Volcanic Hazards, 221–256, Springer. Gorini, M. A. and Bryan, G. M. (1976) The tectonic fabric of Equatorial Atlantic and adjoining continental margins: Gulf of Guinea to northeastern Brazil. Anais da Academia Brasileira de Ciências 48, 10–119. Graham, D. (2002) Noble gas isotope geochemistry of midoceanic ridge and ocean island basalts: characterization of mantle source reservoirs. Noble Gases in Geochemistry and Cosmochemistry (Porcelli, D., Ballentine, C. J. and Wieler, R., eds.), Rev. Mineral. Geochem. 47, 247–317, Geochem. Soc. Mineral. Soc. America, Washington, D.C. Halbwachs, M. and Sabroux, J.-C. (2001) Removing CO2 from Lake Nyos in Cameroon. Science 292, 436. Halbwachs, M., Grangeon, J., Sabroux, J.-C. and Villevielle, A. (1993) Purge par auto-siphon du gaz carbonique dissous dans le lac Monoun (Cameroun): premiers resultats experimentaux. C.R. Acad. Sci., Paris 316, Series II, 483– 489. Halbwachs, M., Sabroux, J.-C., Grangeon, J., Kayser, J. G., Tochon-Danguy, J.-C., Felix, A., Beard, J.-C., Villevieille, A., Vitter, G., Richon, B., Wuest, A. and Hell, J. (2004) Degassing the “Killer Lakes” Nyos and Monoun, Cameroon. EOS 85(30), 281–288. Halliday, A. N., Dickin, A. P., Fallick, A. E. and Fitton, J. G. (1988) Mantle dynamics: a Nd, Sr, Pb and O isotopic study of the Cameroon line volcanic chain. J. Petrol. 29, 181– 211. Halliday, A. N., Davidson, J. P., Holden, P., DeWolf, C., Lee, D.-C. and Fitton, J. G. (1990) Trace-element fractionation in plumes and the origin of HIMU mantle beneath the Cameroon line. Nature 347, 523–528. Hart, S. R. (1984) A large-scale isotope anomaly in the Southern Hemisphere mantle. Nature 309, 753–757. Holloway, J. R. and Blank, J. G. (1994) Application of experimental results to C-O-H species in natural melts. Volatiles in Magmas, Rev. Mineralogy 30, 187–230, Mineral Soc. Amer. Igarashi, G., Ozima, M., Ishibashi, J., Gamo, T., Sakai, H., Nojiri, Y. and Kawai, T. (1992) Mantle helium flux from the bottom of Lake Mashu, Japan. Earth Planet. Sci. Lett. 108, 11–18. Ihinger, P. D., Hervig, R. L. and McMillan, P. F. (1994) Analytical methods for volatiles in glasses. Volatiles in Magmas, Rev. Mineralogy 30, 67–121, Mineral Soc. Amer. Issa, Ohba, T., Fantong, W., Fouepe, A., Chako Tchamabé, B., Yoshida, Y., Kusakabe, M., Sigha, N., Tsunogai, U., Oginuma, Y., Tanyileke, G., Satake, H. and Hell, J. V. (2013) Contribution of methane to total gas pressure in deep waters at lakes Nyos and Monoun (Cameroon, West Africa). Geochem. J. 44, 349–362. Issa, Fantong, W. Y., Aka, F. T., Ohba, T., Chako Tchamabé, B., Rouwet, D., Yoshida, Y., Gbetnkom Mouliom, A., Sighomnoun, D., Sigha, N., Kusakabe, M., Tanyileke, G. and Hell, J. V. (2014a) d18O and dD variation in some volcanic lakes along the Cameroon Volcanic Line (WestAfrica): Generating an isotopic baseline data for volcanoes monitoring/surveillance in Cameroon. J. Limnol. 74, 95–113, doi:10.4081/jlimnol.2014.966. Issa, Ohba, T., Chako Tchamabé, B., Padrón, E., Hernández, P., Eneke Takem, E. G., Barrancos, J., Sighomnoun, D., Ooki, S., Sigha, N., Kusakabe, M. and Yoshida, Y. (2014b) doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 Gas emission from some Diffuse Diffusion Structures (DDSs) of the Cameroon Volcanic Line (CVL): Implication for the prevention of CO2 related hazards. J. Volcanol. Geotherm. Res. 283, 82–93, doi:10.1016/ j.jvolgeores.2014.07.001. Johnson, M. C., Anderson, A. T., Jr. and Rutherford, M. J. (1994) Pre-eruptive volatile contents of magmas. Volatiles in Magmas, Rev. Mineralogy 30, 281–330, Mineral Soc. Amer. Joint UNEP/OCHA Environment Unit (2005) Lake Nyos Dam Assessment. Embedded in http://www.reliefweb.int/ library/documents/2005/ocha-cmr Kamgang, P., Chazot, G., Njonfang, E., Ngongang, N. B. T. and Tchoua, F. M. (2013) Mantle sources and magma evolution beneath the Cameroon Volcanic Line: geochemistry of mafic rocks from the Bamenda Mountains (NW Cameroon). Gondwana Res. 24, 727–741. Kamtchueng, B. T., Fantong, W. Y., Ueda, A., Tiodjio, E. R., Anazawa, K., Wirmvem, M. J., Mvondo, J. O., Nkamdjou, L. S., Kusakabe, M., Ohba, T., Tanyileke, G. and Hell, J. V. (2014) Assessment of shallow groundwater in Lake Nyos catchment (Cameroon, Central-Africa): implications for hydrogeochemical controls and uses. Environ Earth Sci. 72, 3663–3678, doi:10.1007/s12665-014-3278-6. Kamtchueng, B. T., Fantong, W. Y., Tiodjio, E. R., Takounjou, A. F., Wirmvem, M. J., Djomou, S. L. B., Asai, K., Kusakabe, M., Ohba, T., Tanyileke, G., Hell, J. V. and Ueda, A. (2015a) A multi-tracer approach for assessing the origin, apparent age and recharge mechanism of shallow groundwater in the Lake Nyos catchment, Northwest, Cameroon. J. Hydrol. 523, 790–803, doi:10.1016/j.jhydrol. 2015.02.008. Kamtchueng, B. T., Fantong, W. Y., Takounjou, A. F., Tiodjio, E. R., Kusakabe, M., Mvondo, J. O., Zhang, J., Ohba, T., Tanyileke, G., Hell, J. V. and Ueda, A. (2015b) Hydrogeochemistry of surface- and groundwater in the vicinity of Lake Monoun, West Cameroon: Approach from multivariate statistical analysis and stable isotopic characterization. Environ. Monitor. Assessment Jour. (in press). Kanari, S. (1989) An inference on process of gas outburst from lake Nyos, Cameroon. J. Volcanol. Geotherm. Res. 39, 135–149. Kantha, L. H. and Freeth, S. J. (1996) A numerical simulation of the evolution of temperature and CO2 stratification in Lake Nyos since the 1986 disaster. J. Geophys. Res. 101(B4), 8187–8203. Kerrick, D. M. (2001) Present and past nonanthropogenic CO 2 degassing from the solid Earth. Earth. Rev. Geophys. 39, 565–585. Kipfer, R., Aeschbach-Hertig, W., Baur, H., Hofer, M., Imboden, D. M. and Signer, P. (1994) Injection of mantle type helium into Lake Van (Turkey): the clue for quantifying deep water renewal. Earth Planet. Sci. Lett. 125, 357–370. Kipfer, R., Aeschbah-Hertig, W., Peeters, F. and Stute, M. (2002) Noble gases in lakes and ground waters. Noble Gases in Geochemistry and Cosmochemistry (Porcelli, D., Ballentine, C. J. and Wieler, R., eds.), Rev. Mineral. Geochem. 47, 615–700, Mineral. Soc. America, Washington, D.C. Kling, G. W. (1988) Comparative transparency, depth of mixing, and stability of stratification in lakes of Cameroon, 47 West Africa. Limnol. Oceanogr. 33, 27–40. Kling, G. W., Clark, M. A., Compton, H. R., Devine, J. D., Evans, W. C., Humphrey, A. M., Koenigsberg, E. J., Lockwood, J. P., Tuttle, M. L. and Wagner, G. N. (1987) The 1986 Lake Nyos gas disaster in Cameroon, West Africa. Science 236, 169–175. Kling, G. W., Evans, W. C., Tuttle, M. L. and Tanyileke, G. (1994) Degassing of Lake Nyos. Nature 368, 405–406. Kling, G. W., Evans, W. C., Tanyileke, G., Kusakabe, M., Ohba, T., Yoshida, Y. and Hell, J. V. (2005) Degassing Lakes Nyos and Monoun: Defusing certain disaster. Proc. Nat. Acad. Sci. USA 102, 14185–14190. Kozono, T., Kusakabe, M., Yoshida, Y., Ntchantcho, R., Ohba, T., Tanyileke, G. and Hell, J. V. (2016) Numerical assessment of the potential for future limnic eruptions at lakes Nyos and Monoun, Cameroon, based on regular monitoring data. Geochemistry and Geophysics of Active Volcanic Lakes (Ohba, T., Capaccioni, B. and Caudron, C., eds.), Geological Society, London, Special Publications, 437 (http://doi.org/10.1144/SP437.8). Kusakabe, M. (2015) Evolution of CO2 content in Lakes Nyos and Monoun, and sub-lacustrine CO2-recharge system at Lake Nyos as envisaged from CO 2/3He ratios and noble gas signatures. Volcanic Lakes (Rouwet, D., Christenson, B., Tassi, F. and Vandemeulebrouck, J., eds.), 427–450, Springer-Verlag, Berlin, Heidelberg, doi:10.1007/978-3-642-36833-2_19. Kusakabe, M. and Sano, Y. (1992) Origin of gases in Lake Nyos, Cameroon. Natural Hazards in West and Central Africa, International Monograph Series on Interdisciplinary Earth Science Research and Applications (Freeth, S. J., Ofoegb, C. O. and Onohua, K. M., eds.), 83–95, Friedrich Vieweg & Sohn Verlag, Braunschweig, Wiesbaden. Kusakabe, M., Ohsumi, T. and Aramaki, S. (1989) The Lake Nyos gas disaster: chemical and isotopic evidence in waters and dissolved gases from three Cameroonian crater lakes, Nyos, Monoun and Wum. J. Volcanol. Geotherm. Res. 39, 167–185. Kusakabe, M., Tanyileke, G., McCord, S. A. and Schladow, S. G. (2000) Recent pH and CO2 profiles at Lakes Nyos and Monoun, Cameroon: implications for the degassing strategy and its numerical simulation. J. Volcanol. Geotherm. Res. 97, 241–260. Kusakabe, M., Ohba, T., Issa, Yoshida, Y., Satake, H., Ohizumi, T., Evans, W. C., Tanyileke, G. and Kling, G. W. (2008) Evolution of CO 2 in Lakes Monoun and Nyos, Cameroon, before and during controlled degassing. Geochem. J. 42, 93–118. Kusakabe, M., Ohba, T., Yoshida, Y., Anazawa, K., Kaneko, K., Ueda, A. and Miyabuchi, Y. (2011) Lake Nyos gas disaster (Cameroon): Latest situation. The 8th Annual Meeting of Asia Oceania Geosciences Society (AOGS2011), Taipei, 8–12 August, 2011. Abstract ID: SE52-A002. Le Guern, F., Tazieff, H. and Faivre Pierret, R. (1982) An example of health hazard: People killed by gas during a phreatic eruption: Dieng Plateau (Java, Indonesia), February 20th 1979. Bull. Volcanol. 45(2), 153–156. Le Guern, F., Shanklin, E. and Tebor, S. (1992) Witness accounts of the catastrophic event of August 1986 at Lake Nyos (Cameroon). J. Volcanol. Geotherm. Res. 51, 171– 184. doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. 48 M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 Lee, D. C., Halliday, A. N., Fitton, J. F. and Poli, G. (1994) Isotopic variation with distance and time in the volcanic Islands of the Cameroon line: evidence for a mantle plume origin. Earth Planet. Sci. Lett. 123, 119–138. Lee, D. C., Halliday, A. N., Davies, G. R., Essene, E. J., Fitton, J. G. and Temdjim, R. (1996) Melt enrichment of shallow depleted mantle: a detailed petrological, trace element and isotopic study of mantle-derived xenoliths and megacrysts from the Cameroon Line. J. Petrol. 37, 415– 441. Lockwood, J. P. and Rubin, M. (1989) Origin and age of the Lake Nyos maar, Cameroon. J. Volcanol. Geotherm. Res. 39, 117–124. Lockwood, J. P., Costa, J. E., Tuttle, M. L., Nni, J. and Tebor, S. G. (1988) The potential for catastrophic dam failure at Lake Nyos maar, Cameroon. Bull. Volcanol. 50, 340–349. Marty, B. and Jambon, A. (1987) C/3He in volatile fluxes from the solid Earth: implications for carbon geodynamics. Earth Planet. Sci. Lett. 83, 16–26. Marzoli, A., Peccirillo, E. M., Renne, P. R., Bellieni, G., Iacumin, M., Nyobe, J. B. and Tongwa, A. T. (2000) The Cameroon Volcanic Line revisited: petrogenesis of continental basaltic magmas from lithospheric and asthenospheric mantle sources. J. Petrol. 41, 87–109. Marzoli, A., Aka, F. T., Merle, R., Callegaro, S. and N’ni, J. (2015) Deep to shallow crustal differentiation of withinplate alkaline magmatism at Mt. Bambouto volcano, Cameroon Line. Lithos 220–223, 272–288. Mbassa, B. J., Njonfang, E., Benoit, M., Kamgang, P., Grégoire, M., Duchene, S., Brunet, P., Ateba, B. and Tchoua, F. M. (2012) Mineralogy, geochemistry and petrogenesis of the recent magmatic formations from Mbengwi, a continental sector of the Cameroon Volcanic Line (CVL), Central Africa. Mineral. Petrol. 106, 217– 242. McCord, S. A. and Schladow, S. G. (1998) Numerical simulations of degassing scenarios for CO 2-rich Lake Nyos, Cameroon. J. Geophys. Res. 103, 12355–12364. Meyers, J., Rosendalh, B. R., Harrison, C. G. and Ding, Z. D. (1998) Deep-imaging seismic and gravity results from the offshore Cameroon Volcanic Line, and speculation of African hotlines. Tectonophysics 284, 31–63. Molua, E. L. and Lambi, C. M. (2006) Climate, Hydrology and Water Resources in Cameroon. Centre for Environment Economics and Plicy in Africa (CEEPA), Pretoria, 37 pp. Moreau, C., Regnoult, J. M., Deruelle, B. and Robineau, B. (1987) A new tectonic model for the Cameroon Line, Central Africa. Tectonophysics 139, 317–334. Morgan, W. J. (1983) Hotspot tracks and the early rifting of the Atlantic. Tectonophysics 94, 123–139. Nagao, K., Kusakabe, M., Yoshida, Y. and Tanyileke, G. (2010) Noble gases in Lakes Nyos and Monoun, Cameroon. Geochem. J. 44, 519–54. Nkoumbou, C., Deruelle, B. and Velde, D. (1995) Petrology of Mt. Etinde Nephelinite series. J. Petrol. 36, 373–395. Nkouandou, O. F. and Temdjim, R. (2011) Petrology of spinel lherzolite xenoliths and host basaltic lava from Ngao Voglar volcano, Adamawa Massif (Cameroon Volcanic Line, West Africa): equilibrium conditions and mantle characteristics. J. Geosci. 56, 375–387. Nojiri, Y., Kawai, T. and Otsuki, A. (1990) Estimation of lake water mixing from the distribution of temperature, conductivity and dissolved chemical constituents in Lake Mshu. Res. Rep. Natl. Inst. Environ. Stud., Japan No. 126, 25–65 (in Japanese). Nojiri, Y., Kusakabe, M., Tietze, K., Hirabayashi, J., Sato, H., Sano, Y., Shinohara, H., Njine, T. and Tanyileke, G. (1993) An estimate of CO 2 flux in Lake Nyos, Cameroon. Limnol. Oceanogr. 38, 739–752. Ohba, T., Ooki, S., Oginuma, Y., Kusakabe, M., Yoshida, Y., Ueda, A., Anazawa, K., Saiki, K., Kaneko, K., Miyabuchi, Y., Issa, Aka, F., Fantong, W., Ako, A., Tanyileke, G. and Hell, J. V. (2016) Decreasing removal rate of the dissolved CO 2 in Lake Nyos, after the installation of additional degassing pipes. Geochemistry and Geophysics of Active Volcanic Lakes (Ohba, T., Capaccioni, B. and Caudron, C., eds.), Geological Society, London, Special Publications 437 (http://doi.org/10.1144/SP437.6). Ohsumi, T., Nakashiki, N., Shitashima, K. and Hirama, K. (1992) Density change of water due to dissolution of carbon dioxide and near-field behavior of CO 2 from a source on deep-sea floor. Energy Convers. Mgmt. 33, 685–690. Palme, H. and O’Neill, H. (2003) Cosmochemical Estimates of mantle composition. The Mantle and Core (Carlson, R. W., ed.), In Treatise on Geochemistry, Vol. 2 (Holland, H. D. and Turekian, K. K., eds.), 1–38, Elsevier-Pergamon, Oxford. Pasternak, G. B, and Varekamp, J. C. (1997) Volcanic lake systematics I. Physical constraints. Bull. Volcanol. 58, 528–538. Pouclet, A., Dongmo, A. K., Bardintzeff, J. M., Wandji, P., Tagheu, P. C., Nkouathio, D., Bellon, H. and Ruffet, G. (2014) The Mount Manengouba, a complex volcano of the Cameroon Line: Volcanic history, petrological and geochemical features. J. Afr. Earth Sci. 97, 297–321. Rankenburg, K., Lassiter, J. C. and Brey, G. (2005) The role of continental crust and lithospheric mantle in the genesis of Cameroon Volcanic Line lavas: constraints from isotopic variations in lavas and megacrysts from Biu and Jos Plateaux. J. Petrol. 46(1), 169–190. Reusch, A. M., Nyblade, A. A., Wiens, D. A., Shore, P. J., Ateba, B., Tabod, C. T. and Nnange, J. M. (2010) Upper mantle structure beneath Cameroon from body wave tomography and the origin of the Cameroon Volcanic Line. Geochem. Geophys. Geosyst. 11, issue 10, doi:10.1029/ 2010GC003200. Rozanski, K., Araguas, L. and Gonfiantini, R. (1993) Isotope patterns in modern global precipitation. Climate Change in Continental Isotopic Records (Swart, P. K., Lohmann, K. C., Mckenzie, J. and Savin, S., eds.), Geophys. Mongr. Ser. 78, 1–36, AGU, Washington, D.C. Saiki, K., Kaneko, K., Ohba, T., Sanemasa, M., Kusakabe, M., Ntchancho, R., Fouepe, A., Tanileke, G. and Hell, J. V. (2016) Vertical distribution of dissolved CO2 from lakes Nyos and Monoun (Cameroon) as estimated by sound speed in water. Geochemistry and Geophysics of Active Volcanic Lakes (Caudron, C., Capaccino, B. and Ohba, T. eds.), Geological Society, London, Special Publications 437 (http://doi.org/10.1144/SP437.10). Sano, Y. and Williams, S. N. (1996) Fluxes of mantle and subducted carbon along convergent plate boundaries. Geophys. Res. Lett. 23, 2749–2752. Sano, Y., Wakita, H., Ohsumi, T. and Kusakabe, M. (1987) doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 Helium isotope evidence for magmatic gases in Lake Nyos, Cameroon. Geophys. Res. Lett. 14, 1039–1041. Sano, Y., Kusakabe, M., Hirabayashi, J., Nojiri, Y., Shinohara, H., Njine, T. and Tanyileke, G. (1990) Helium and carbon fluxes in Lake Nyos, Cameroon: constraint on next gas burst. Earth Planet. Sci. Lett. 99, 303–314. Schmid, M., Lorke, A., Wuest, A., Halbwachs, M. and Tanyileke, G. (2003) Development and sensitivity analysis of a model for assessing stratification and safety of Lake Nyos during artificial dagassing. Ocean Dynamics 53, 288–301. Schmid, M., Lorke, A., Dinkel, C., Tanyileke, G. and Wuest, A. (2004) Double-diffusive convection in Lake Nyos, Cameroon. Deep-Sea Res. I 51, 1097–1111. Schmid, M., Halbwachs, M., Wehrli, B. and Wüest, A. (2005) Weak mixing in Lake Kivu: New insights indicate increasing risk of uncontrolled gas eruption. Geochem. Geophys. Geosyst. 6, doi:10.1029/2004GC000892. Schmid, M., Halbwachs, M. and Wuest, A. (2006) Simulation of CO 2 concentrations, temperature, and stratification in Lake Nyos for different degassing scenarios. Geochem. Geophys. Geosyst. 7, doi:10.1029/ 2005GC001164. Schoell, M., Tietze, K. and Schoberth, S. (1988) Origin of the methane in Lake Kivu (east-central Africa). Chem. Geol. 71, 257–265. Shanklin, E. (1989) Exploding lakes and maleficent water in Grassfields legends and myth. J. Volcanol. Geotherm. Res. 39, 233–246. Shanklin, E. (1992) Natural disasters in the oral history of West Cameroon. Natural Hazards in West and Central Africa (Freeth, S. J., Ofoegbu, C. O. and Onuoha, K. M., eds.), 57–62, Friedr. Vieweg & Sohn Verlag, Braunschweig, Wiesbaden. Shanklin, E. (2007) Exploding lakes in myth and reality: an African case study. Geological Society, London, Special Publications 273, 165–176, doi:10.1144/ GSL.SP.2007.273.01.14. Shinohara, H. (2003) Chemical phenomena induced by crustal fluids. Chikyukagaku Koza No. 3 (Geochemistry of Earth’s Mantle and Crust) (Notsu, K. and Shimizu, H., eds.), 271–295, Baifu-kan, Tokyo (in Japanese). Sigurdsson, H. (1987a) Lethal gas bursts from Cameroon crater lakes. EOS 68, 570–573. Sigurdsson, H. (1987b) A dead chief’s revenge? Natural History 8, 44–49. Sigurdsson, H., Devine, J. D., Tchoua, F. M., Presser, T. S., Pringle, M. K. and Evans, W. C. (1987) Origin of the lethal gas burst from Lake Monoun, Cameroun. J. Volcanol. Geotherm. Res. 31, 1–16. Sigvaldason, G. E. (1989) International Conference on Lake Nyos Disaster, Yaounde, Cameroon 16–20 March 1987: Conclusions and Recommendations. J. Volcanol. Geotherm. Res. 39, 97–107. Sorey, M. L., Evans, W. C., Kennedy, B. M., Farrar, C. D., Hainsworth, L. J. and Hausback, B. (1998) Carbon dioxide and helium emissions from a reservoir of magmatic gas beneath Mammoth Mountain, California. J. Geophys. Res. 103, 15303–15323. Stager, C. and Suau, A. (1987) Silent death from Cameroon’s killer lake. National Geographic September 1987, 404– 420. 49 Staudacher, T. and Allègre, C. J. (1988) Recycling of oceanic crust and sediments. The noble gas subduction barrier. Earth Planet. Sci. Lett. 89, 173–183. Suh, C. E., Sparks, R. S. J., Fitton, J. G. and Ayonghe, S. N. (2003) The 1999 and 2000 eruptions of Mount Cameroon: eruption behavior and petrochemistry of lava. Bull. Volcanol. 65, 267–281. Tassi, F., Vaselli, O., Tedesco, D., Montegrossi, G., Darrah, T., Cuoco, E., Mapendano, M. Y., Poreda, R. and Delgado Huertas, A. (2009) Water and gas chemistry at Lake Kivu (DRC): Geochemical evidence of vertical and horizontal heterogeneities in a multibasin structure. Geochem. Geophys. Geosyst. 10(1), doi:10.1029/2008GC002191. Tazieff, H. (1989) Mechanisms of the Nyos carbon dioxide disaster and of so-called phreatic steam eruptions. J. Volcanol. Geotherm. Res. 39, 109–116. Tedesco, D., Vaselli, O., Papale, P., Carn, S. A., Voltaggio, M., Sawyer, G. M., Durieux, J., Kasereka, M. and Tassi, F. (2007) January 2002 volcano-tectonic eruption of Nyiragongo volcano, Democratic Republic of Congo. J. Geophys. Res. 112(B9), doi:10.1029/2006JB004762. Tedesco, D., Tassi, F., Vaselli, O., Poreda, R. J., Darrah, T., Cuoco, E. and Yalire, M. M. (2010) Gas isotopic signatures (He, C, and Ar) in the Lake Kivu region (western branch of the East African rift system): Geodynamic and volcanological implications. J. Geophys. Res. 115, B01205, doi:10.1029/2008JB006227. Thomas, L. E., Howkesworth, C. J., Van Calsteren, P., Turner, S. P. and Rogers, N. W. (1999) Melt generation beneath ocean islands: a U-Th-Ra isotopic study from Lanzarote in the Canary Islands. Geochim. Cosmochim. Acta 63, 4081–4099. Tietze, K. (1987) The Lake Nyos gas catastrophe in Cameroon: cause, sequence of events, consequences. Proc. XXII Congress IAHR, Topics in Lake and Reservoir Hydraulics (Graf, W. H., ed.). Tietze, K. (1992) Cyclic gas bursts: are they a ‘usual’ feature of Lake Nyos and other gas-bearing lakes? Natural Hazards in West and Central Africa (Freeth, S. J., Ofoegbu, C. O. and Onuoha, K. M., eds.), 97–107, Fridr. Vieweg, Braunschweig/Wiesbaden. Tietze, K., Geyh, M., Müller, H., Schröder, L., Stahl, W. and Wehner, H. (1980) The genesis of the methane in Lake Kivu (Central Africa). Geol. Rundsch. 69, 452–472, doi:10.1007/BF02104549. Tiodjio, R. E., Sakatoku, A., Nakamura, A., Tanaka, D., Fantong, W. Y., Kamtchueng B. T., Tanyileke, G., Ohba, T., Hell, J. V., Kusakabe, M, Nakamura, S. and Ueda, A. (2014) Bacterial and archaeal communities in Lake Nyos (Cameroon, Central Africa). Sci. Rep. 4, 6151, doi:10.1038/srep06151. Tiodjio, R. E., Fantong, W. Y., Kamtchueng, B. T., Tanyileke, G., Ohba, T., Hell, V. J., Kusakabe, M., Nakamura, S. and Ueda, A. (2015) Bacteriological assessment of drinking water sources in the vicinities of Lakes Nyos and Monoun (Cameroon, Central Africa). J. Environ. Sci. Water Res. 4(3), 60–70. Tiodjio, R. E., Sakatoku, A., Issa, Fantong, W. Y., Tchakam, K. B., Tanyileke, G., Hell, V. J., Ohba, T., Kusakabe, M., Tanaka, D., Nakamura, S. and Ueda, A. (2016) Vertical distribution of bacteria and archaea in a CO 2 -rich meromictic lake: a case study of Lake Monoun. doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. 50 M. Kusakabe / GEochem. Monogr. Ser. 1: 1–50, 2017 Limnologica (in press). Tsafack, T. J. P., Wandji, P., Bardintzeff, J. M., Bellon, H. and Guillou, H. (2009) The Mount Cameroon stratovolcano (Cameroon volcanic line, Central Africa): petrology, geochemistry, isotope and age data. Geochem., Mineral. Petrol. SOFIA 47, 65–78. VOLADA (2013) https://vhub.org/resources/2822 Yokoyama, T., Aka, F. T., Kusakabe, M. and Nakamura, E. (2007) Plume-lithospheric interaction beneath Mt. Cameroon volcano, West Africa: Constraints from 238U230 Th- 226Ra and Sr-Nd-Pb isotope systematics. Geochim. Cosmochim. Acta 71, 1835–1854. Yoshida, Y., Issa, Kusakabe, M., Satake, H. and Ohba, T. (2010) An efficient method for measuring CO2 concentration in gassy lakes: Application to Lakes Nyos and Monoun, Cameroon. Geochem. J. 44, 441–448. Yoshida, Y., Kusakabe, M., Issa, Ohba, T., Tanyileke, G. and Hell, J. V. (2016) Decreasing capability of the degassing systems at Lakes Nyos and Monoun (Cameroon): A proposal for a new system aiming at prevention of recurrence of a future limnic eruption. Geochemistry and Geophysics of Active Volcanic Lakes (Caudron, C., Capaccino, B. and Ohba, T., eds.), Geological Society, London, Special Publications 437 (http://doi.org/10.1144/SP437.3). doi:10.5047/gems.2017.00101.0001 © 2017 TERRAPUB, Tokyo. All rights reserved. View publication stats