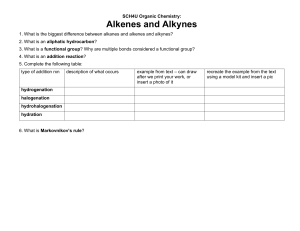
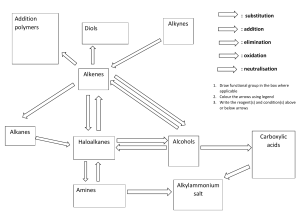
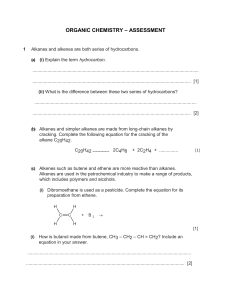
Hydrocarbons Objectives: • Draw and name hydrocarbons as a prelude to naming other types of organic compounds; and • Give some properties and functions of different hydrocarbons. Aliphatic vs. Aromatic Aliphatic Hydrocarbons Cyclic Hydrocarbons Molecular Representations of Compounds Alkanes • • • • • described as saturated hydrocarbon contain the maximum possible number of hydrogens per carbon Only contains single bond Formula: CnH2n+2, where n is any integer Names end with a suffix -ane Straight-Chain Alkanes Branched-Chain Alkanes Cyclic Alkanes Nomenclature of Alkanes • In the early nineteenth century, organic compounds were often named at the whim of their discoverers. IUPAC Nomenclature • Systematic names devised by the International Union of Pure and Applied Chemistry (IUPAC) Naming Straight-Chain Alkanes Naming Branched-chain Alkanes Example: Example: 1. 2. Naming Complex Substituents • Treat the complex substituent as if it is a miniparent with its own substituents. • Begin by placing numbers on the substituent, going away from the parent chain. • Place parentheses around the name of the substituent. Common Names Practice Problems: c. a. b. Activity 1 (Nomenclature of Alkanes) 1. c. b. 2. Cycloalkanes • compounds with rings of carbon atoms • represented by polygons • general formula (CH2)n, or CnH2n Naming Cycloalkanes Practice Problems: Unsaturated Hydrocarbons Alkenes • contains at least one C=C bond • general formula of CnH2n, where n is any integer ethene Alkynes • contains at least one C C bond • general formula of CnH2n-2, where n is any integer ethyne Naming Straight-Chain Alkenes & Alkynes Name of Alkene, Alkyne ethene, ethyne propene, propyne butene, butyne pentene, pentyne hexene, hexyne heptene, heptyne octene, octyne nonene, nonyne decene, decyne Naming Branched Chain Alkenes & Alkynes Naming Cycloalkenes Practice Problems: Practice Problems: Properties of Hydrocarbons Alkanes Alkenes • generally unreactive • Non-polar • Insoluble in water, soluble in most organic solvents • Melting point, boiling point and density increases as the no. of carbon atoms increases. • reactive than alkanes • Non-polar • Insoluble in water, soluble in most organic solvents • Melting point, boiling point and density increases as the no. of carbon atoms increases. Alkynes • more reactive than alkenes • Non-polar • Insoluble in water, soluble in most organic solvents • Melting point, boiling point and density increases as the no. of carbon atoms increases. Uses of Alkanes • First four carbons – used for heating, cooking and electricity generation • Carbon Number 5-8 – are volatile liquids, good solvents for nonpolar substances • Carbon Number 9-16 – form the major part of diesel and aviation fuel • Carbon No. 17 and upwards – components of fuel oil and lubricating oil also used as anti-corrosive agents, paraffin wax, used for road surfacing Uses of Alkenes Uses of Alkyne • used as fuel for welding metals (ethyne) • starting material in the synthesis of polymers