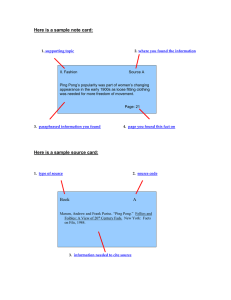
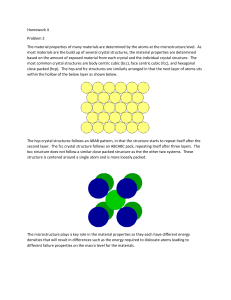
Last Name Section Number First Name Instructor Name ENGR1600: Materials Science for Engineers Interactive Activity 03 CRYSTAL STRUCTURE ACTIVITY AND QUESTIONS Objective: To build an understanding of how atoms pack together in common crystal structures. Note: There is no prelab procedure required for this Activity. Materials: Aluminum plate with holes (you might have to share plates) 4 pieces of 10 inch long wooden blocks 60 white ping pong balls. 8 orange ping pong balls Mathematica CDF App (download “Crystal Structures” from LMS) Smart Phone Fcc, bcc, hcp models Procedure: Tip 1: build the crystal layer by layer, with the help of either wood blocks (parts A, C, D) or aluminum plate (part B) to restrict the positions of the balls. Tip 2: You will need pictures from all parts before your leave the classroom. Anything else you do not finish can be completed on your own time. A. Using four wooden blocks and ping pong balls, build a simple cubic extended lattice (several unit cells). Do not use aluminum plate here. Take a picture using a smart phone, then label three unit cell vectors on the picture. Insert the picture into the picture table as part of the report. Use the CDF app, show simple cubic crystal, with the unit cell drawn. Take a screenshot. How many atoms are there per unit cell? ______ What is the coordination number of each atom? _________ Write an equation describing the relationship between a, the lattice parameter, and r, the atomic radius. ___________________ B. Using an aluminum plate only (no wooden blocks), create a 4x4 layer of white ping pong balls. Add a 3x3 layer, etc. until you have built a pyramid. Can you identify the crystal structure?_______ Once you have identified the unit cell rebuild your structure using 8 orange balls to clearly show the vertices (corners) of a single unit cell in your model. Take a picture using a smart phone, then label three unit cell vectors on the picture. Label the closest packed plane and a close packed direction on the picture. Insert the picture into the picture table as part of the report. 1 Use the CDF app, select the correct crystal, with the unit cell drawn. Use draw planes to indicate the closest packed plane. Take a screenshot. How many atoms are there per unit cell? ______ What is the coordination number of each atom? _________ Write an equation describing the relationship between a, the lattice parameter, and r, the atomic radius. C. Using three wooden blocks and ping pong balls, build one close-packed layer at a time. Devise a strategy to make a face centered cubic (fcc) crystal 4 layers high. Hint: build a triangle with 7 ping pong balls per side for your bottom layer. This is a (111) plane of atoms What is the stacking sequence that should be used? _______ Use 8 orange balls (as cube vertices) to identify the conventional unit cell of FCC. Take a picture using a smart phone, then label three unit cell vectors (of the conventional unit cell), Label the close-packed plane, a close packed direction on the picture. Insert the picture into the picture table as part of the report. Tip: the picture needs to show the correct stacking (be mindful of the viewing angle, and you may need to remove some atoms selectively) Use the CDF app, select FCC crystal, with the unit cell drawn. Use “draw planes” to indicate the closest packed plane. Take a screenshot. How many atoms are there per unit cell? ______ What is the coordination number of each atom? _________ Write an equation describing the relationship between a, the lattice parameter, and r, the atomic radius. _____________________________ D. using the same bottom layer as in C, make an HCP crystal. What is the stacking sequence that should be used? __________ Take a picture using a smart phone. Insert the picture into the table as part of the report. Tip: the picture needs to show the correct stacking (be mindful of the viewing angle, and you may need to remove some atoms selectively) Use the CDF app, select HCP crystal. Take a screenshot. IMPORTANT NOTES: 1) PLEASE PUT AWAY ALL OF YOUR PING PONG BALLS INTO THE STORAGE BOX 2) Turn in the answers to ALL questions and your photographs via LMS in a PDF file. . POST LAB REPORT: Download this MSWord file, insert your photos, screenshots, and answers to the above questions. When you are ready to upload, save file as PDF and go to “TURN IN LAB REPORT” to left of LMS page. (To check for due date, go to “TURN IN LAB REPORT” on left of LMS screen). 2 PICTURE TEMPLATE (Be sure to follow instructions in labeling from above) Also, you can share the cell phone picture, but each individual must label the image by themselves and grab a screenshot from the Mathematica activity by themselves. Mathematica CFD screenshot D (HCP) C (FCC) B (___) A (SC) Ping Pong ball model 3