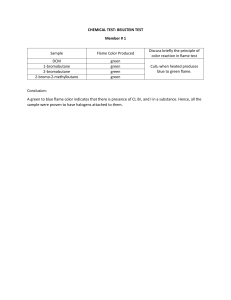
Annasaheb Dange College of Engineering & Technology, Ashta (An Autonomous Institute)
Chapter 2: INSTRUMENTAL METHODS OF CHEMICAL ANALYSIS
I. Advantages and Disadvantages of Instrumental Methods:
Advantages/Merits of Instrumental Methods:
1.
2.
2.
3.
4.
5.
Small quantities of samples (in micro scale) can be analyzed.
These methods are easy to handle.
Instruments are highly sensitive and the results are very accurate.
The results are reliable.
Instrumental methods are very fast.
Complex, coloured, turbid and colloidal samples can be analysed and
handled.
6. Poisonous and Carcinogenic samples can be analyzes by using these
methods.
7. Most of the instruments are compatible with printer and other devices.
So, data can be stored, printed and managed easily.
Disadvantages/De- Merits (Limitations) of Instrumental Methods:
1. Everytime calibration and standardization is required before going to
use. e.g.- pH-Meter is standardized by using buffer solutions.
2. Sensitivity and accuracy varies with different make of the instrument.
3. The cost of the instrument/equipment is high.
4. Special training is required to operate the instrument.
II. Glass electrode:
a) Principle: When a glass surface is kept in contact with a solution, a
potential is developed, called as glass electrode potential.
The value of this potential is the function of H+ ion concentration and
the nature of the glass electrode. The glass electrode potential (GEP) is
given by: EG = E0G - 0.0591 log [H+]
pH meter
Glass
Electrode
Platinum
Wire
Calomel
electrode
0.1N
HCl
Solution of
Unknown
Measurement of pH with glass electrode
Notes by- Mr. Z. D. Sande, Department of Basic Sciences
Annasaheb Dange College of Engineering & Technology, Ashta (An Autonomous Institute)
b) Construction of Glass Electrode: The glass electrode consists of a glass
bulb with long neck. The bulb is made from a special type of low melting glass.
A solution of 0.1N HCl saturated with quinhydrone is filled in the bulb. A
platinum wire is inserted into the solution for making the electrical contact.
c) Working: To find out the pH with glass electrode, the glass bulb is inserted
in an unknown solution and then it is combined with a standard calomel
electrode.
The cell can be represented as,
- Pt | 0.1N HCl |Glass |Unknown Solution || SCE +
The e.m.f. of the cell is given as,
Ecell = Eright - Eleft
Ecell = ESCE - {E0G - 2.303 RT/nF log [H+]}
Ecell = ESCE - { E0G - 0.0591 log [H+] }
Ecell = ESCE - {E0G + 0.0591 pH}
Ecell = ESCE - E0G - 0.0591 pH
pH =
ESCE - Ecell - E0G
0.0591
But, ESCE = 0.2422 and E0G is also known for the glass electrode.
pH =
0.2422 - Ecell - E0G
0.0591
Where, EoG is standard glass electrode potential. It is constant for the given
electrode and its value depends on the nature and composition of the glass. The value
of EoG is determined by measuring the e.m.f. of the buffer solution with known pH
value.
d) Advantages and disadvantages:
Advantages:
1.
2.
3.
4.
5.
6.
7.
8.
It is unaffected by the oxidation - reduction potentials.
It can be used both in alkaline and oxidizing agents.
It is useful to measure pH between 0 to 10.
With special type of glass pH up to 14 can be measured.
Glass electrode is very simple to operate.
It comes to equilibrium very quickly.
It can be used in coloured, turbid and colloidal solutions.
They are strong to withstand moderately severe treatment.
Notes by- Mr. Z. D. Sande, Department of Basic Sciences
Annasaheb Dange College of Engineering & Technology, Ashta (An Autonomous Institute)
Disadvantages:
1. The bulb is very fragile and therefore great care is required for its
use.
2. The glass electrode has high electrical resistance.
3. It cannot be used with ordinary potentiometers, but can be used
only with electronic potentiometers.
4. Standardization is required every time when it is to be used.
5. It cannot be used in pure ethyl alcohol, acetic acid and gelatin.
III. SPECTROMETRY:
Laws of spectrometry:
a) Lambert’s law: (Applicable for Solid materials). Lambert has given the
relation between the extent of light absorbed and the thickness of the medium or
absorbing material.
According to Lambert’s law, when a beam of monochromatic light passes
through a transparent medium, then the rate of decrease of intensity of light with
the thickness of the absorbing medium is proportional to the intensity of the
incident light.
Mathematically, Lambert’s law can be stated as,
dI I
dt
dI kI
dt
dI
- kdt ……………………………...
I
(1)
Where, I = Intensity of the incident light of wavelength .
dI = The decrease of intensity when light passes through a medium of
thickness dt and k = The proportionality constant called as ‘absorption coefficient’. It depends up on the wavelength of incident light and the nature of
the absorbing medium.
If,
Io = Intensity of incident light before entering in the medium i.e. when t = 0
and
It = The intensity of the light after passing through a medium of thickness t,
then by integrating the equation (1), we get,
It
dI
I =
Io
t t
- k.dt
t 0
loge It/ Io = - k t
-kt
It / I o = e
-0.4343kt
t/ Io = 10
-t
t/ Io = 10
-t
t = Io . 10 ………………………………… (2)
Notes by- Mr. Z. D. Sande, Department of Basic Sciences
Annasaheb Dange College of Engineering & Technology, Ashta (An Autonomous Institute)
Where, = 0.4343k and it is called as ‘absorption co-efficient’ of the
substance.
If, It = 0.1 = 10-1 and Io = 1 then,
-1
= 10-t
αt = 1
α
Hence, the absorption co-efficient α is defined as the reciprocal of the
thickness t, required to reduce the light to 1/10th of its intensity.
DEFINATIONS:
1) Transmittance (T): It is defined as the fraction of the incident light
transmitted by a thickness t of the medium.
T = It /Io
2) Absorbance (A): The logarithm of the reciprocal of the transmittance is
called as absorbance (A) or optical density (D).
A = Loge
Io
It
= log
Intensity of incidentlight
Intensity of transmitte d light
b) Beer’s (Beer-Lamberts law): When the absorbing medium is in the form
of solution, then the Beer’s law is applicable.
According to Beer’s law: When a beam of monochromatic light passes
through a solution of absorbing substance, the rate of decrease of intensity of
light with the thickness of the solution is proportional to the intensity of the
incident light and the concentration of the solution.
Mathematically, the law can be stated as,
dI c I
dt
dI k' c I
dt
dI
- k' c dt ……………….………….
I
(3)
Where, c = concentration of the solution in moles/liter and
k’ = proportionality constant called as molar absorption co-efficient.
It depends upon the wavelength of the light and the nature of the absorbing substance.
If,
Io = Intensity of the incident light before entering in the solution i.e. when t =
0 and
It = the intensity of the light after passing through a solution of thickness t,
Then, by integrating the equation (3) we get,
It
t t
Io
t 0
dI
I = - k'.c.dt
Notes by- Mr. Z. D. Sande, Department of Basic Sciences
Annasaheb Dange College of Engineering & Technology, Ashta (An Autonomous Institute)
Log
It
- k' c t
I0
It
e - k' c t
I0
It
10- 0.4343k' c t
I0
It
e- ε c t
Io
∴ It = Io . 10- εct
(4)
Where, = 0.4343k’ and it is called as ‘molar extinction co-efficient’ of the
substance. The above equation is Lambert’s- Beer’s law or simply Beer’s law.
From this equation we have,
It
10- ε c t
Io
Io
10- ε c t
It
Io
log ε c t
It
But,
log Io/It = D or A.
A = ε c t or D = ε c t ……………………… (5)
If, c = 1 mole/liter and t = 1 cm
Then,
D = ε ,or A = ε
Hence, the molar extinction co-efficient can be defined as the optical density,
when the solution has the thickness 1cm and the concentration of the solution is
one mole/liter.
Single beam spectrophotometer:
The schematic representation of single beam spectrophotometer is as shown in the
figure,
A) Construction:
The single beam spectrophotometer consists of the following parts:
1. Source of light: A
strong incandescent light
source (Tungston Filament)
emitting continuous spectrum
of light is used.
2. Monochromator:
The prism or grating is used
as monochromator which
allows only a narrow beam of
monochromatic light.
M
Source
1
S
M
P
S
1
S
1
2
M
3
2
S
S
S
Monochromator
3
4
D
Detector
Figure: Single beam spectrophotometer
Notes by- Mr. Z. D. Sande, Department of Basic Sciences
Annasaheb Dange College of Engineering & Technology, Ashta (An Autonomous Institute)
3. Detector: A photovoltaic cell with amplifier and galvanometer are used to
measure the intensity of transmitted light. The photomultiplier tubes are also used as
detector.
B) Working:
The light from the source S is condensed by the mirror M1, it passes through
slit S1, incidents on mirror M2 and reflected towards monochromator through slit S2.
The beam of light is further reflected by mirror M3 in such a way that parallel
beam falls on the prism P.
The prism reflects back the dispersed beam on mirror M3 which reflects it
towards sample S. When it passes through slit S3 it is almost monochromatic. The
transmitted light then passes through slit S4 and fall on the detector D.
C) Concentration of unknown solution: The concentration of unknown solution can
be determined with the help of spectrometers.
For example,
If
As
Au
Cs
Cu
= Absorbance of standard solution,
= Absorbance of unknown solution,
= Concentration of standard solution,
= Concentration of unknown solution
Then,
As = ε
or
As
εt
Cs
Au = ε
or
Au
εt
Cu
and
As Au
=
Cs Cu
or
Cu =
Au x Cs
As
If the absorbance Au of unknown solution is measured and the values of the Cs
and As for standard solution are known, then concentration of unknown solution Cu
can be calculated.
Notes by- Mr. Z. D. Sande, Department of Basic Sciences
Annasaheb Dange College of Engineering & Technology, Ashta (An Autonomous Institute)
FLAME PHOTOMETRY:
(BASIC CONCEPT, INSTRUMENTATION, AND APPLICATIONS)
INTRODUCTION:
i) Flame photometry (more accurately called Flame Atomic Emission
Spectrometry)is a branch of spectroscopy in which the species examined in the
spectrometer are in the form of atoms.
ii) A photoelectric flame photometer is an instrument used in inorganic chemical
analysis to determine the concentration of certain metal ions among them sodium,
potassium, calcium and lithium.
iii) Flame Photometry is based on measurement of intensity of the light emitted when
a metal is introduced into flame. – The wavelength of colour tells what the
element is (qualitative) – The colour's intensity tells us how much of the element
present (quantitative) change back to ground state.
iv) When a metal salt solution is burned, the metal provides a colored flame and each
metal ion gives a different colored flame.
v) Flame tests, therefore, can be used to test for the absence or presence of a metal
ion, release energy as photons of particular wavelength, become unstable at high
energy level, specific quantum of thermal energy absorbed by orbital electrons,
subjected to hot flame.
vi) The basic principle upon which Atomic Spectroscopy works is based on the fact
that "Matter absorbs light at the same wavelength at which it emits light".
BASIC CONCEPT: Liquid sample containing metal salt solution is introduced into
a flame solvent is a first vaporized leaving particle of solid salt which is then
vaporized into gaseous state. Gaseous molecule dissociate to give neutral atoms
which can be excited (made unstable) by thermal energy of flame. The unstable
excited atoms emit photons while returning to lower energy state. The measurement
of emitted photons forms the basis of flame photometry.
Under constant and controlled conditions, the light intensity of the characteristic
wavelength produced by each of the atoms is directly proportional to the number of
atoms that are emitting energy, which in turn is directly proportional to the
concentration of the substance of interest in the sample. Various metals emit a
characteristic colour of light when heated.
Structure of Flame: As seen in the figure, the flame may be divided into the
following regions or zones. – Preheating zones – Primary reaction zone or inner zone
– Internal zone – Secondary reaction zone.
Notes by- Mr. Z. D. Sande, Department of Basic Sciences
Annasaheb Dange College of Engineering & Technology, Ashta (An Autonomous Institute)
Schematic appearance of flame
Preheating zone- In this, combustion mixture is heated to the ignition
temperature by thermal conduction from the primary reaction zone.
i)
Primary reaction zone- This zone is about 0.1 mm thick at atmospheric
pressure – There is no thermodynamic equilibrium in this zone and the concentration
of ions and free radicals is very high. This region is not used for flame photometry.
ii)
Interconal zone – It can extend up to considerable height. The maximum
temperature is achieved just above the tip of the inner zone. – This zone is used for
flame photometry.
iii) Secondary reaction zone - In this zone, the products of the combustion
processes are burnt to stable molecular species by the surrounding air.
INSTRUMENTATION: THE FLAME PHOTOMETER:
A general scheme of a Flame Photometer
1) Sample Delivery System: There are three components for introducing liquid
sample:
a) Nebulizer – it breaks up the liquid into small droplets. Nebulization is
conversion of a sample to a mist of finely divided droplets using a jet of
compressed gas. – The flow carries the sample into the atomization region.
– Pneumatic Nebulizers: (most common).
Notes by- Mr. Z. D. Sande, Department of Basic Sciences
Annasaheb Dange College of Engineering & Technology, Ashta (An Autonomous Institute)
b) Aerosol modifier – it removes large droplets from the stream and allow
only smaller droplets than a certain size to pass.
c) Flame or Atomizer – it converts the analyte into free atoms.
2) Source: A Burner used to spray the sample solution into fine droplets. Several
burners and fuel+oxidant combinations have been used to produce analytical
flame including: Premixed, Mecker, Total consumption, Lundergarh, Shielded
burner, and Nitrous oxide-acetylene flames.
Pre-mixed Burner: Widely used because uniformity in flame intensity.
In this energy type of burner, aspirated sample, fuel and oxidant are thoroughly
mixed before reaching the burner opening.
Total Consumption Burner: – In this fuel and oxidant are hydrogen and
oxygen gases Sample solution is aspirated through a capillary by high pressure
of fuel and Oxidant and burnt at the tip of burner entire sample is consumed.
3) Monochromator: – Prism: Quartz material is used for making prism, as quartz
is transparent over entire region – Grating: it employs a grating which is
essentially a series of parallel straight lines cut into a plane surface.
4) Detectors: – Photomultiplier tubes – Photo emissive cell – Photo voltaic cell
Photovoltaic cell: •
It has a thin metallic layer coated with silver or gold which act as
electrode, also has metal base plate which act as another electrode. Two layers
are separated by semiconductor layer of selenium, when light radiation falls on
selenium layer. This creates potential diff. between the two electrode and cause
flow of current.
5) Read-out Device: It is capable of displaying the absorption spectrum as well
absorbance at specific wavelength. Nowadays the instruments have
microprocessor controlled electronics that provides outputs compatible with
the printers and computers. Thereby minimizing the possibility of operator
error in transferring data.
APPLICATIONS:
a. To estimate sodium, potassium, calcium, lithium etc. level in sample of
serum, urine, CSF and other body fluids.
b. Flame photometry is useful for the determination of alkali and alkaline earth
metals.
c. It is used in determination of lead in petrol. I
d. t is used in the study of equilibrium constants involving in ion exchange
resins.
e. It is used in determination of calcium and magnesium in cement.
IV. CHROMATOGRAPHY:
Definition:
It is the method of separating a mixture of components into individual
components by equilibrium distribution between the two phases.
Notes by- Mr. Z. D. Sande, Department of Basic Sciences
Annasaheb Dange College of Engineering & Technology, Ashta (An Autonomous Institute)
OR
A technique of separation and purification of components of a mixture by
their differing affinities for two phases (states) of a matter with which they come
into contact.
This technique was first discovered by Russian Botanist- Mikhail Tswett in
1903 for separation of plant pigments such as chlorophyll. Later on, Martin and Synge
developed various techniques.
PRINCIPLE: In any chromatographic technique, the components of a mixture
to be separated are distributed between two phases:
a) Stationary Phase: It may be in the form of Solid or Liquid.
For ex- Finely powdered Alumina, Silica or a non-volatile liquid.
It adsorbs the components from solution (Mobile phase).
b) Mobile (Moving) Phase: It may be in the form of liquid or
gas.
For ex- Any organic solvent or an inert gas.
GAS- LIQUID CHROMATOGRAPHY (GLC):
Discovered by- Martin and Synge in 1941.
In this technique the stationary phase is a non- volatile liquid adsorbed on an
inert solid support (Porous) and mobile phase is an Inert Gas like Helium,
Argon, Hydrogen, CO, N2 etc. Different components of a mixture will get
separated depending upon their affinities with the stationary and moving phase.
Notes by- Mr. Z. D. Sande, Department of Basic Sciences
Annasaheb Dange College of Engineering & Technology, Ashta (An Autonomous Institute)
High Performance Liquid Chromatography (HPLC)
Introduction
HPLC is an abbreviation for High Performance Liquid Chromatography (It has also
been referred to as High Pressure LC). Beginning of the 60’s: start of HPLC as High Pressure
Liquid Chromatography and is the largest separations technique used.
Principle:
HPLC is a separation technique that involves the injection of a small volume of liquid
sample into a tube packed with tiny particles (3 to 5 micron (μm) in diameter called the
stationary phase). Where individual components of the sample are moved down the packed
tube (column) with a liquid (mobile phase) forced through the column by high pressure
delivered by a pump.
In principle, LC and HPLC work the same way except the speed, efficiency, sensitivity
and ease of operation of HPLC is vastly superior. These components are separated from one
another by the column packing that involves various chemical and/or physical interactions
between their molecules and the packing particles.
These separated components are detected at the exit of this tube (column) by a flowthrough device (detector) that measures their amount. An output from this detector is called a
“liquid chromatogram”.
Instrumentation
A flow scheme for HPLC
As shown in the schematic diagram in Figure above, HPLC instrumentation includes a
pump, injector, column, detector and integrator or acquisition and display system. The heart
of the system is the column where separation occurs.
1. Solvent Reservoir: Mobile phase contents are contained in a glass reservoir. The mobile
phase, or solvent, in HPLC is usually a mixture of polar and non-polar liquid components
whose respective concentrations are varied depending on the composition of the sample.
2. Pump: A pump aspirates the mobile phase from the solvent reservoir and forces it
through the system’s column and detector. Depending on a number of factors including
column dimensions, particle size of the stationary phase, the flow rate and composition of
the mobile phase, operating pressures of up to 42000 kPa (about 6000 psi) can be
generated.
Notes by- Mr. Z. D. Sande, Department of Basic Sciences
Annasaheb Dange College of Engineering & Technology, Ashta (An Autonomous Institute)
3. Columns:- Columns are usually made of polished stainless steel, are between 50 and 300
mm long and have an internal diameter of between 2 and 5mm long. They are commonly
filled with a stationary phase with a particle size of 3–10 µm. Columns with internal
diameters of less than 2 mm are often referred to as microbore columns. Ideally the
temperature of the mobile phase and the column should be kept constant during an
analysis.
4. Detector:- The HPLC detector, located at the end of the column detect the analytes as
they elute from the chromatographic column. Commonly used detectors are UVspectroscopy, fluorescence, mass-spectrometric and electrochemical detectors.
5. Data Collection Devices: Signals from the detector may be collected on chart recorders
or electronic integrators that vary in complexity and in their ability to process, store and
reprocess chromatographic data. The computer integrates the response of the detector to
each component and places it into a chromatograph that is easy to read and interpret.
APPLICATION OF HPLC (USES):
The information that obtained by HPLC includes resolution, identification and
quantification of a compound. It also aids in chemical separation and purification.
The other applications of HPLC include1) Pharmaceutical Applications- To control drug stability. Tablet dissolution study of
pharmaceutical dosages form. Pharmaceutical quality control.
2) Environmental Applications- Detection of phenolic compounds in drinking water.
Bio-monitoring of pollutants.
3) Applications in Forensics- Quantification of drugs in biological samples.
Identification of steroids in blood, urine etc. Forensic analysis of textile dyes.
4) Food and Flavor- Measurement of Quality of soft drinks and water. Sugar analysis in
fruit juices. Analysis of polycyclic compounds in vegetables. Preservatives analysis.
QUESTION BANK:
Q-1) Explain working of single beam spectrometer with suitable diagram. How it can be used
to determine the concentration of unknown solution?
Q-2) Explain the principle, construction & working of glass electrode.
Q-3) Write short notes ona) Beer-Lambert’s law/ Beer’s Law.
b) Construction & working of glass electrode.
c) Lambert’s law.
d) Applications of pH-metry.
e) Advantages of instrumental methods over Non-instrumental methods.
Q-4) Write any four applications of pH-metry.
Q-5) Explain the principle, construction & working of GLASS ELECTRODE. What are its
advantages & disadvantages?
Q-6) What are advantages and disadvantages of glass electrode?
Q-7) Explain construction & working of single beam spectrometer.
Q-8) What is Chromatography? Give the classification of chromatography.
Q-9) What is GLC? Explain principle, instrumentation and working of GLC.
Q-10) What are the applications of GLC?
Notes by- Mr. Z. D. Sande, Department of Basic Sciences
Annasaheb Dange College of Engineering & Technology, Ashta (An Autonomous Institute)
Q-11) Explain the principle, construction & working of Flame Photometry/Flame Atomic
Emission Spectrometry. What are it’s applications.
Q-12) Explain the principle, construction & working of HPLC. What are it’s applications.
Notes by- Mr. Z. D. Sande, Department of Basic Sciences