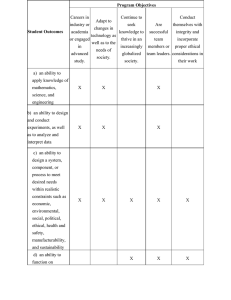
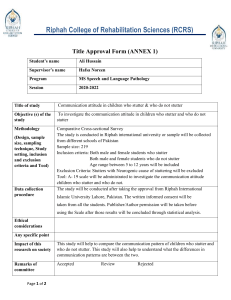
Riphah College of Rehabilitation & Allied Health Sciences (RCRS) Title Approval Form (ANNEX 1) Student’s name Supervisor’s name Program Session Title of study Objective (s) of the study Methodology (Design, sample size, sampling technique, Study setting, inclusion and exclusion criteria and Tool) Data collection procedure Ethical considerations Approval will be gained from the Ethical committee of the Riphah international university Lahore, Pakistan prior to the commencement of study. Written informed consent will be taken from all the patients and all information and data will be confidential. Subjects will be informed that there is no risk of study and they will be free to withdraw any time during process of study. Any specific point Impact of this research on society Remarks of committee Accepted Signature of the committee: Director RCRS: Member of Committee: Member of Committee: Page 1 of 2 Review Rejected Page 2 of 2