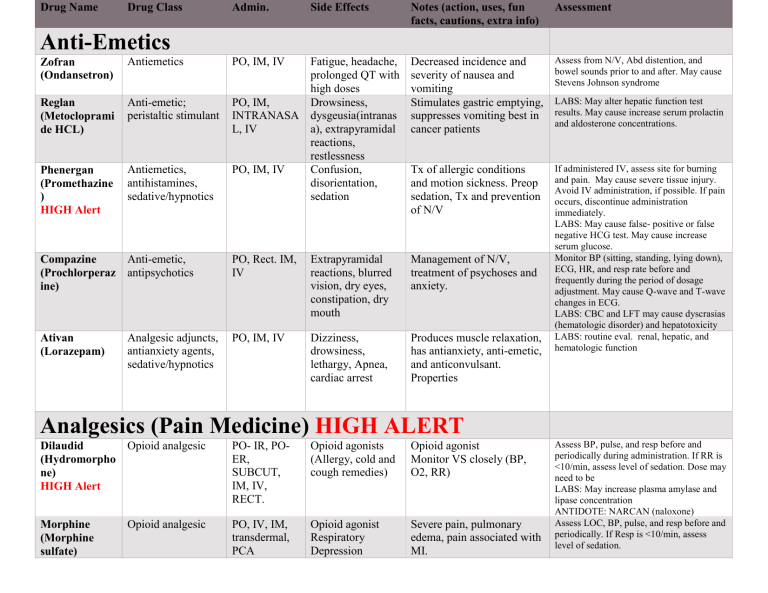
Drug Name Drug Class Admin. Side Effects Notes (action, uses, fun facts, cautions, extra info) Assessment Fatigue, headache, prolonged QT with high doses PO, IM, Drowsiness, INTRANASA dysgeusia(intranas L, IV a), extrapyramidal reactions, restlessness PO, IM, IV Confusion, disorientation, sedation Decreased incidence and severity of nausea and vomiting Stimulates gastric emptying, suppresses vomiting best in cancer patients Assess from N/V, Abd distention, and bowel sounds prior to and after. May cause Stevens Johnson syndrome Tx of allergic conditions and motion sickness. Preop sedation, Tx and prevention of N/V PO, Rect. IM, IV Extrapyramidal reactions, blurred vision, dry eyes, constipation, dry mouth Management of N/V, treatment of psychoses and anxiety. PO, IM, IV Dizziness, drowsiness, lethargy, Apnea, cardiac arrest Produces muscle relaxation, has antianxiety, anti-emetic, and anticonvulsant. Properties If administered IV, assess site for burning and pain. May cause severe tissue injury. Avoid IV administration, if possible. If pain occurs, discontinue administration immediately. LABS: May cause false- positive or false negative HCG test. May cause increase serum glucose. Monitor BP (sitting, standing, lying down), ECG, HR, and resp rate before and frequently during the period of dosage adjustment. May cause Q-wave and T-wave changes in ECG. LABS: CBC and LFT may cause dyscrasias (hematologic disorder) and hepatotoxicity LABS: routine eval. renal, hepatic, and hematologic function Anti-Emetics Zofran (Ondansetron) Antiemetics Reglan (Metocloprami de HCL) Anti-emetic; peristaltic stimulant Phenergan (Promethazine ) HIGH Alert Antiemetics, antihistamines, sedative/hypnotics Anti-emetic, Compazine (Prochlorperaz antipsychotics ine) Ativan (Lorazepam) Analgesic adjuncts, antianxiety agents, sedative/hypnotics PO, IM, IV LABS: May alter hepatic function test results. May cause increase serum prolactin and aldosterone concentrations. Analgesics (Pain Medicine) HIGH ALERT Opioid analgesic Dilaudid (Hydromorpho ne) HIGH Alert PO- IR, POER, SUBCUT, IM, IV, RECT. Opioid agonists (Allergy, cold and cough remedies) Opioid agonist Monitor VS closely (BP, O2, RR) Opioid analgesic PO, IV, IM, transdermal, PCA Opioid agonist Respiratory Depression Severe pain, pulmonary edema, pain associated with MI. Morphine (Morphine sulfate) Assess BP, pulse, and resp before and periodically during administration. If RR is <10/min, assess level of sedation. Dose may need to be LABS: May increase plasma amylase and lipase concentration ANTIDOTE: NARCAN (naloxone) Assess LOC, BP, pulse, and resp before and periodically. If Resp is <10/min, assess level of sedation. HIGH Alert Hypotension, constipation, confusion, sedation Sedation, respiratory depression, constipation Confusion, dizziness, sedation, hypotension, constipation, dyspepsia, nausea Respiratory depression, confusion, sedation, constipation. Respiratory depression, nausea, vomiting, itching ANTIDOTE: NARCAN (naloxone) Fentanyl (Fentanyl Citrate) HIGH ALERT Norco/Vicodin (Hydrocodone) HIGH Alert Opioid analgesic IM, IV Decrease pain, supplement in anesthesia Opioid analgesic PO, PO-ER Roxicodone (Oxycodone) HIGH Alert Opioid analgesic PO, PO-ER Percocet (Oxycodone) HIGH Alert Opioid analgesic PO, PO-ER Tylenol-3 (Tylenol with Codeine) HIGH Alert Opioid analgesics PO Hypotension, nausea, vomiting, confusion, sedation Opioid combined with acetaminophen antipyretics, Tylenol (Acetaminophe nonopioid analgesics n/APAP) PO, IV, rectal Nausea, tiredness, vomiting, paleness Mild pain, fever NSAID Ibuprofen (Advil, Motrin) PO, IV H/A, constipation, dyspepsia, N/V Take with food to prevent GI upset. Pain and anti-inflammatory, fever Opioid combined with acetaminophen (Tylenol) Monitor RR and BP may cause sleep related breathing disorders. s/s of toxicity- resp depression, hypotension, arrythmias, bradycardia. ANTIDOTE: NARCAN (naloxone) Assess BP, pulse, and resp before and periodically during administration. If RR is <10/min, assess level of sedation. LABS: May increase plasma amylase and lipase concentration ANTIDOTE: NARCAN (naloxone) Decrease moderate to severe Assess BP, pulse, and resp before and periodically during administration. If RR is pain Decrease moderate to severe pain <10/min, assess level of sedation. LABS: May increase plasma amylase and lipase concentration ANTIDOTE: NARCAN (naloxone) Assess BP, pulse, and resp before and periodically during administration. If RR is <10/min, assess level of sedation. LABS: May increase plasma amylase and lipase concentration ANTIDOTE: NARCAN (naloxone) Assess BP, pulse, and resp before and periodically during administration. If RR is <10/min, assess level of sedation. LABS: May increase plasma amylase and lipase concentration ANTIDOTE: NARCAN (naloxone) LABS: Increased serum bilirubin, LDH, AST, ALT, and PT may indicate hepatotoxicity. Toxicity Overdose: acetylcysteine (Acetadote) LABS: BUN, Serum CREA, CBC, Gabapentin (Neurontin) Analgesic adjuncts, therapeutic, anticonvulsants, mood stabilizers PO- IR, POSR Dizziness, Partial seizures, postherpetic vasodilation, neuralgia, RLS. tremors, dry mouth Ketamine General anesthetics IV, IM Emergence reactions, HTN, tachycardia ONLY TO BE GIVEN BY MD Lidocaine Analgesic/local anesthetic IV, IM, Local Confusion, drowsiness Control of ventricular arrhythmias, local anesthesia Narcan Opioid Antagonist IV, IM, Subcut, Intranasal Reverses opioid drug affects, increase pain Reversal of signs of opioid excess Toradol (Ketorolac tromethamine) Nonopioid analgesics PO, IM, IV, IN Drowsiness May increase GI bleeding (avoid with these patients) Works amazing for kidney stone pain PO, IV Constipation, dizziness, difficulty sleeping, headache, diarrhea. PO, PO-ER, IV, topical, vaginal Dizziness, H/A, stomach upset, N/V, loss of appetite, diarrhea. Used for sinusitis, PNA, anthrax, UTI Broad spectrum for Gram () and Gram (+) as well as atypical respiratory infections PO: Tx of anaerobic infections IV: perioperative prophylactic agent in colorectal surgery Vag: management of bacterial vaginosis Monitor closely for notable changes in behavior that could indicate the emergence or worsening of suicidal thoughts or behavior or depression. LABS: False- positive readings when testing urine protein. May cause liver dysfunction with recurrent use. TOXICITY: Respiratory depression or apnea may be treated with mechanical ventilation or analeptics. Monitor ECG continuously and BP and Respiratory LABS: Serum electrolyte levels, IM adminis. May cause increase CK levels Monitor respiratory rate, rhythm, and depth; pulse, ECG, BP; and level of consciousness frequently for 3–4 hr after the expected peak of blood concentrations. Minimal toxicity Evaluate liver function tests, especially AST and ALT. May cause ↑ levels. May cause prolonged bleeding time that may persist for 24–48 hr following discontinuation of therapy. May cause ↑ BUN, serum creatinine, or potassium concentrations Antibiotics Levaquin (Levofloxacin) Fluoroquinolone Antibiotic Anti-infectives, Flagyl (Metronidazole antiprotozoals, antiulcer agents ) LABS: May cause ↑ serum AST, ALT, LDH, bilirubin, and alkaline phosphatase. May also cause ↑ or ↓ serum glucose. LABS: May alter results of serum AST, ALT, and LDH tests Nausea, diarrhea, vomiting, dyspepsia, tendon rupture Pain at IM site, Phlebitis at IV site Inhibits bacterial DNA synthesis by inhibiting DNA gyrase enzyme. LABS: May cause ↑ serum AST, ALT, LDH, bilirubin, and alkaline phosphatase. May also cause ↑ or ↓ serum glucose. Bactericidal action against susceptible bacteria PO Diarrhea, N/V or upset stomach Anti- infectives IV, PO Nephrotoxicity, phlebitis Used in respiratory, GI, GU. Breaks down Gram (-) bacteria; breaks down cell wall Bactericidal action against susceptible organisms Tetracyclines Tetracycline Antibiotic PO Diarrhea, N/V, photosensitivity Bacteriostatic action against suspectable bacteria Doxycycline (Vibramycin – Doxy calcium) Anti-infectives PO, IV Diarrhea, N/V, photosensitivity Augmentin (Amoxicillinclavulanate potassium) Anti-infectives PO Diarrhea, rash Used in PNA, STD, malaria, anthrax, periodontitis. Gram (+) and (-) Encourage taking with meal to decrease GI sx Bactericidal action against susceptible bacteria. Streptococci, Pneumococci, Enterococci, Haemophilus influenzae, Escherichia coli, Proteus mirabilis, Neisseria meningitidis,N. gonorrhoeae, LABS: May cause positive results for Coombs' test. May cause increased AST, ALT, alkaline phosphatase, bilirubin, LDH, BUN, and serum creatinine. LABS: May cause positive results for Coombs' test. May cause increased AST, ALT, alkaline phosphatase, bilirubin, LDH, BUN, and serum creatinine. LABS: Monitor for casts, albumin, or cells in the urine or decreased specific gravity, CBC, and renal function periodically during therapy. May cause increased BUN levels. TOXICITY: Trough concentration should not exceed 10mcg/mL or 15-20mcg/mL May cause ↑ AST, ALT, serum alkaline phosphatase, bilirubin, and amylase concentrations. Tetracyclines, except doxycycline, may cause elevated serum BUN. May cause false ↑ in urinary catecholamine levels. Monitor renal and hepatic functions and CBC periodically during long-term therapy. May cause ↑ AST, ALT, serum alkaline phosphatase, bilirubin, and amylase concentrations. May cause false ↑ in urinary catecholamine levels. LABS: May cause ↑ serum alkaline phosphatase, LDH, AST, and ALT concentrations. Elderly men and patients receiving prolonged treatment are at ↑ risk for hepatic dysfunction. May cause false-positive direct Coombs' test result. Ciprofloxacin Anti-infectives PO, PO-ER, IV Rocephin (Ceftriaxone) Cephalosporin Antibiotic IM, IV Keflex Anti-infectives Vancomycin Bactrim (Sulfamethoxa zoletrimethoprim) Sulfonamide-folate antagonist Antibiotic PO, IV Penicillin V Anti- infectives PO, IV, IM Zosyn (Piperacillin) Anti-infectives IV agents for atypical Zithromax (Azithromycin) mycobacterium, anti-infectives IV, PO Staphylococcus aureus, Klebsiella pneumoniae, Shigella, Salmonella, Moraxella catarrhalis. Phlebitis at IV site, Active against many strains loss of appetite, of gram-positive aerobic N/V, dizziness pathogens including: Streptococcus pneumoniae, Staphylococcus aureus, Group A beta-hemolytic streptococci, Nocardia, Enterococcus. Diarrhea, rash, Active against: epigastric distress, Most gram-positive N/V organisms, including many streptococci and Bacillus anthracis, Some gram-negative organisms, such as Neisseria meningitidis and N. gonorrhoeae Some anaerobic bacteria and spirochetes including Borellia burgdorferi. GI upset, oral or Appendicitis and peritonitis. vaginal Skin and skin structure candidiasis, rash, infections. anaphylaxis Gynecologic infections. Community-acquired and nosocomial pneumonia. Abd. Pain Treatment Diarrhea, nausea Upper respiratory tract infections (streptococcal pharyngitis, acute bacterial exacerbations of chronic bronchitis and tonsillitis) LABS: Monitor CBC and urinalysis periodically during therapy. May produce ↑ serum bilirubin, ↑ potassium, creatinine, and alkaline phosphatase. May cause hypoglycemia. May cause positive direct Coombs' test results. May cause ↑ AST, ALT, LDH, and serum alkaline phosphatase concentrations. May cause leukopenia and neutropenia, especially with prolonged therapy or hepatic impairment. Evaluate renal and hepatic function, CBC, serum potassium, and bleeding times prior to and routinely during therapy. LABS: May cause increase serum bili, AST , ALT, LDH, and alkaline phosphatase concentration Lower respiratory tract infections (bronchitis and pneumonia) Acute otitis media, Skin and skin structure infections, Nongonococcal urethritis, cervicitis, gonorrhea, and chancroid. Prevention of disseminated Mycobacteriu m avium complex (MAC) infection in patients with advanced HIV infection. Treatment of serious gramnegative bacterial infection. And infection caused by. Staphylococcus when penicillin or other less toxic drugs are contraindicated. Gentamicin Aminoglycoside Antibiotic IM, IV Kidney failure, ototoxicity, itching, swelling, rash Ancef (Cefazolin) anti- infectives IV, IM Diarrhea, N/V, rash, pain at IM site, Phlebitis at IV site Used in presurgical, Sepsis, GU tract infections, trauma Used frequently in open fractures Anti- inflammatory IV, PO HTN, acne, decrease wound healing, ecchymoses, hirsutism, petechiae, adrenal suppression, anorexia nausea, muscle wasting, osteoporosis, depression, euphoria Used systemically and locally in a wide variety of chronic diseases including: Inflammatory, Allergic, Hematologic, Endocrine, Neoplastic, Dermatologic, Autoimmune disorders, Management of cerebral edema, Diagnostic agent in adrenal disorders. LABS: Monitor. Renal function by urinalysis, specific gravity, BUN, serum creatinine, and CCR. May cause increase in BUN, AST, alt, serum alkaline Phosphatase, bilirubin, serum creatinine, and LDH concentration. TOXICITY: Trough levels >2mcg/mL May cause increase serum ALT, AST, alkaline phosphatase, bilirubin, LDH, BUN, and serum creatinine. Steroids Decadron (Dexamethaso ne) Monitor serum electrolytes and glucose. May cause hyperglycemia, especially in patients with diabetes. Monitor hematologic values, serum electrolytes, and serum and urine glucose in patients on prolonged therapy. May cause ↓ WBC counts. May cause ↓ serum potassium and calcium and ↑ serum sodium concentrations HTN, acne, decrease wound healing, ecchymoses, fragility hirsutism, petechiae, adrenal suppression, anorexia nausea, muscle wasting, osteoporosis, depression, euphoria Peptic ulcers, GI bleed, hyperglycemia, delayed wound healing, Cushing’s syndrome Suppresses inflammatory and immune system by inhibiting synthesis of mediators Reduces inflammation, redness, warmth, pain Push IV slowly: will cause projectile vomiting Monitor serum electrolytes and glucose. May cause hyperglycemia, especially in persons with diabetes. May cause hypokalemia. Patients on prolonged therapy should routinely have hematologic values, serum electrolytes, and serum and urine glucose evaluated. May ↓ WBC counts. May ↓ serum potassium and calcium and increase serum sodium concentrations Suppression of inflammation and modification of the normal immune response Monitor serum electrolytes and glucose. May cause hyperglycemia, especially in persons with diabetes. May cause hypokalemia. Patients on prolonged courses of therapy should routinely have hematologic values, serum electrolytes, and serum and urine glucose evaluated. May decrease WBC counts. May ↓ serum potassium and calcium and ↑ serum sodium concentrations IV, PO Peptic ulceration, thromboembolism anorexia, nausea, depression, Suppression of inflammation and modification of the normal immune response. Replacement therapy in adrenal insufficiency. Monitor serum electrolytes and glucose. May cause hyperglycemia, especially in persons with diabetes. May cause hypokalemia. Patients on prolonged courses of therapy should routinely have hematologic values, serum electrolytes, and serum electrolytes evaluated. May decrease WBC counts. May decrease serum potassium and calcium and increase serum sodium concentrations. PO, PO-CD,LA, XT IV Arrhythmias, HF, Stevens- Johnson syndrome, peripheral edema Decreases contractility/conductivity, demand for O2 Systemic vasodilation resulting in decreased BP. Coronary vasodilation resulting in decreased Monitor BP, HR, ECG (may cause prolonged PR interval) Monitor intake and output/ daily weight Assess for signs of HF (peripheral edema, rales/crackles, dyspnea, weight gain, jugular venous distention). LABS: Solu-Medrol methylprednis olone Corticosteroid PO, IM, IV Prednisone Antiinflammatories, Immune modifiers PO Cortisone Antiinflammatories Anti-arrhythmic Diltiazem Calcium Channel Blocker frequency and severity of attacks of angina. Reduction of ventricular rate in atrial fibrillation or flutter. Amiodarone HIGH ALERT Antiarrhythmics PO, IV HF, worsening of arrhythmias, QT intervals prolonged. Bradycardia, hypotension, nausea, vomiting, photosensitivity, dizziness, fatigue. Suppression of arrhythmias Rythmol (Propafenone hydrochloride) Antiarrhythmics PO Heart failure, bradycardia, arrythmias, blurry vision, GI upset Suppression of ventricular arrhythmias Lidocaine HIGH Alert Anesthetics (topical/local) Antiarrhythmics IV, IM, Local Seizures, confusion, drowsiness, cardiac arrest, stinging IV to be used only with patient on tele monitor. Blocks sodium channel and slows conduction; reduces automaticity in ventricles, accelerates repolarization Total serum calcium concentrations are not affected by calcium channel blockers. Monitor serum potassium periodically. Hypokalemia ↑ the risk of arrhythmias and should be corrected. Monitor renal and hepatic functions periodically during long-term therapy. May cause ↑ in hepatic enzymes after several days of therapy, which return to normal on discontinuation of therapy. Monitor ECG, HR and rhythm throughout therapy; PR prolongation, slight QRS widening, and T-wave amplitude reduction with T-wave widening and bifurcation may occur. QT prolongation may be associated with worsening of arrhythmias; monitor closely during IV therapy. Report bradycardia or increase in arrhythmias promptly; patients receiving IV therapy may require slowing rate, discontinuing infusion, or inserting a temporary pacemaker. LABS: Monitor Liver and thyroid functions Monitor ECG (may cause PR and QT prolongation), BP, HR, intake, and output. Assess for signs of HF. LABS: May cause ↑ ANA titer, which is usually asymptomatic and reversible. Monitor prothrombin level in patients taking warfarin; may ↑ effects of warfarin. Toxicity Overdose: Signs of toxicity include hypotension, excessive drowsiness, and decreased or abnormal heart rate. Notify health care professional if these signs occur. Monitor ECG continuously and BP and respiratory status frequently during administration. Lab Test Considerations: Serum electrolyte levels should be monitored periodically during prolonged therapy. IM administration may cause ↑ CK levels. Toxicity: Serum lidocaine therapeutic range 1.5- 5mcg/mL S/S: confusion, excitation, blurred or double vision, nausea, vomiting, ringing in ears, Atropine Anticholinergic / Antidysrthymic IM, subcut, IV Tachycardia, blurred vision, dry mouth, urinary hesitancy, drowsiness Digoxin HIGH ALERT Cardiac glycoside Inotrope PO, IM, IV Fatigue, bradycardia, anorexia, nausea, vomiting Arrhythmias Nitroglycerin Vasodilator Antianginals SL/Translingu Dizziness, h/a, al, PO-ER, hypotension, Oint, Patch, tachycardia IV Adenosine (Adenocard) Antiarrhythmics IV Increased heart rate. Decreased GI and respiratory secretions. Reversal of muscarinic effects. May have a spasmolytic action on the biliary and genitourinary tracts. Increased cardiac output (positive inotropic effect) and slowing of the heart rate (negative chronotropic effect) Relief or prevention of anginal attacks. Increased cardiac output. Reduction of BP Arrythmias, facial Restoration of normal sinus flushing, SOB, MI, rhythm Ventricular tremors, twitching, seizures, difficulty breathing, severe dizziness or fainting, and unusually slow heart rate. Assess ECG, BP, HR, Monitor intake and output. Antidote: physostigmine Monitor apical pulse for 1 full min before administering. Withhold dose and notify health care professional if pulse rate is <60 bpm in an adult, <70 bpm in a child, or <90 bpm in an infant. Monitor ECG during IV administration and 6 hr. after each dose. Notify health care professional if bradycardia or new arrhythmias occur. LABS: Therapeutic serum digoxin levels range from 0.5–2 ng/mL, may be drawn 68hrs after a dose Toxicity: s/s Abd pain, anorexia, nausea, vomiting, visual disturbances, bradycardia, and other arrhythmias Tx of life-threatening arrhythmias= digoxin immune Fab Assess location, duration, intensity, and precipitating factors of patient's anginal pain. Monitor BP and pulse before and after administration. Patients receiving IV nitroglycerin require continuous ECG and BP monitoring. Additional hemodynamic parameters may be monitored. LABS: May cause ↑ urine catecholamine and urine vanillylmandelic acid concentrations. Excessive doses may cause ↑ methemoglobin concentrations. May cause falsely ↑ serum cholesterol levels. Monitor heart rate frequently (every 15–30 sec) and ECG continuously during therapy. A short, transient period of 1st-, 2nd-, or 3rd-degree heart block or asystole may occur following injection; usually resolves quickly due to short duration of adenosine. Once conversion to normal sinus rhythm is achieved, transient arrhythmias (premature ventricular contractions, atrial premature contractions, sinus tachycardia, sinus bradycardia, skipped beats, AV nodal block) may occur, but generally last a few sec. Tachycardia, seizures, Stroke Hypertensive/Hypotensive/Rate Control Decreased BP and HR. Decreased frequency of attacks of angina pectoris. Decreased rate of cardiovascular mortality and hospitalization in pt with HR. Systemic vasodilation resulting in decreased BP. Coronary vasodilation resulting in decreased frequency and severity of attacks of angina Monitor BP, ECG, and HR periodically. Monitor intake and output ratios and daily weights. LABS: may cause increase in BUN, Serum lipoprotein, potassium, triglyceride, uric acid, ANA titers, blood sugar levels, serum AKP, LDH, AST, and ALT levels Tachycardia, drug induced lupus syndrome. Used in HTN, HF by lowering BP in HTN PTs and decreased afterload in PTs with HF. Monitor BP and HR, LABS: Monitor BP, ECG, and HR periodically. Monitor intake and output ratios and daily weights. Hypotension, cough, dizziness Decreases peripheral vascular resistance with OUT increasing CO, rate, or contractility Lowering of BP in hypertensive patients. Increased survival and decreased symptoms in patients with heart failure. Assess patient for signs of angioedema (swelling of face, extremities, eyes, lips, tongue, difficulty in swallowing or breathing); may occur at any time during therapy. Discontinue medication and provide supportive care. Monitor BP and HR LABS: Monitor BUN, creatinine, and electrolyte levels periodically. Serum potassium, BUN and creatinine may be ↑, whereas sodium levels may be ↓. If ↑ BUN or serum creatinine concentrations occur, Metoprolol (Lopressor) HIGH Alert Beta Blocker Antihypertensive PO, PO-ER, IV Fatigue, weakness, erectile dysfunction, bradycardia, HF, Pulmonary Edema Cardene (Nicardipine) Calcium Channel Blocker Antihypertensives, antianginals PO, IV Peripheral edema, Arrhythmias, stevens Johnson syndrome, HF Hydralazine (Apresoline) Antihypertensives Vasodilator PO, IM, IV Lisinopril Ace Inhibitor Antihypertensives PO Monitor BP, ECG, and HR periodically. Monitor intake and output ratios and daily weights. May cause Stevens-Johnson Syndrome. LABS: monitor serum K+, hypokalemia increase risk of arrhythmias. Monitor renal and hepatic functions. Increased survival after myocardial infarction. Coreg (Carvedilol) Beta Blocker Antihypertensives PO, PO-CR Stroke, AV block, bradycardia, hypotension, lung edema Decreased heart rate and BP. Improved cardiac output, slowing of the progression of HF and decreased risk of death. Decrease BP and HR. Decreased frequency of attacks of anginal pectoris and prevention of MI Atenolol Beta Blocker Antianginals, antihypertensives PO Fatigue, weakness, ED, Bradycardia, HF, Pulmonary Edema Lotensin (benazepril HCL) ACE inhibitor Antihypertensives PO Headache, hypotension, cough Lowering BP in PTs with HTN Propranolol (Inderal) HIGH ALERT Beta Blocker Antianginals Antiarrhythmics Antihypertensives Vascular headache suppressants PO, PO-ER, IV Fatigue, weakness, ER. Arrhythmias, bradycardia, HF, pulmonary edema, ERYTHEMA MULTIFORME, E XFOLIATIVE DERMATITIS, ST EVENSJOHNSON SYNDROME, TO Beta 1 & 2 adrenergic antagonist Decreases HR, force of contraction, rate of AV conduction Used for angina, hypertension, dysrhythmias dose reduction or withdrawal may be required. May cause hyperkalemia. Monitor CBC periodically during therapy in patients with collagen vascular disease and/or renal disease. May rarely cause slight ↓ in hemoglobin and hematocrit and agranulocytosis. May cause ↑ AST, ALT, alkaline phosphatase, and serum bilirubin. Monitor BP and HR if HR is below 505 adjust dose. Monitor intake and output. LABS: may cause increased BUN, serum lipoprotein, potassium, triglyceride, uric acid levels, ANA titers and Blood sugars. Monitor BP and HR if HR is below 505 adjust dose. Monitor intake and output. LABS: may cause increased BUN, serum lipoprotein, potassium, triglyceride, uric acid levels, ANA titers and Blood sugars. Monitor BP and HR if HR is below 505 adjust dose. Monitor intake and output. S/s of angioedema (swelling of face, extremities, eyes, lips, tongue, difficulty in swallowing or breathing) LABS: Monitor Renal function. May cause increased BUN, serum creatinine. Monitor CBC may cause decrease hemoglobin, leukopenia, and eosinophilia. May cause increase AST, AKP and ALT, serum BILI, uric acid, and glucose Monitor BP and HR. propranolol IV must have continuous ECG monitoring. Monitor intake and output. LABS: May cause ↑ BUN, serum lipoprotein, potassium, triglyceride, and uric acid levels. May cause ↑ ANA titers. May cause ↓ or ↑ in blood glucose levels. In labile diabetic patients, hypoglycemia may be accompanied by precipitous ↑ of BP. Captopril Ace inhibitors Antihypertensives PO XIC EPIDERMAL NECROLYSIS Dizziness, orthostatic hypotension, GI upset, COUGH, headache, Lower BP, reduced symptoms in PTs with HF. Decreased progression of diabetic nephropathy with decreased need for transplantation or dialysis ANGIOEDEMA Losartan (Cozaar) ARB Anti-hypertensive PO diarrhea, blurred vision, difficulty breathing, dizzy, faintness., fast or irregular heartbeat, nausea or vomiting. Lowering of BP in HTN patients. Decrease progression of diabetic. Nephropathy. Decrease incidence of stroke in patients with hypertension and left ventricular Hypertrophy. Valsartan (Diovan) Antihypertensives PO Hypotension, dizziness, GI issues Amlodipine Antihypertensives (Calcium channel blockers) PO, IV Peripheral edema, hypotension, bradycardia Lowers blood pressure. Decreases risk of heart failure related hospitalizations in patients with heart failure. Decreases risk of death from cardiovascular causes in patients with left ventricular systolic dysfunction following myocardial infarction. Reduction in BP. Decreased frequency and severity of attacks of angina. Reduction in risk of hospitalizations for angina or coronary revascularization in patients with recent documented coronary artery disease. Monitor blood pressure and pulse frequently during. Therapy. Assess patient for signs of angioedema. LABS: monitor renal function. May cause increased B UN and creatinine. May cause hyperkalemia. May cause increased AST, ALT, alkaline phosphatase, and. Bilirubin. Monitor CBC. Every two weeks for the first three months. Assess BP and HR frequently during initial dose. Assess patient for signs of angioedema. LABS: Monitor renal function. May increase BUN and creatinine. May cause increase AST, ALT, and bilirubin. May cause hyperkalemia. May cause slight decrease in hemoglobin and hematocrit. Assess BP and HR frequently during initial dose. Assess patient for signs of angioedema. LABS: monitor renal function. Just going to come in the next day may cause increase be UN and. Serum creatinine. May cause hyperkalemia. May cause increased AST and ALT. May cause slight decrease in hemoglobin and hematocrit or neutropenia. Monitor BP and pulse before therapy, during dose titration, and periodically during therapy. Monitor ECG periodically during prolonged therapy. Monitor intake and output. LABS: Total serum calcium concentration are not affected by calcium channel blockers. Nifedipine Antianginals, antihypertensives (Calcium channel blockers) Po, PO-ER Hypotension, bradycardia, AV block, headache, GI upset, peripheral edema Systemic vasodilation, resulting in decreased BP. Coronary vasodilation, resulting in decrease frequency and severity of attacks of angina Verapamil Antianginals, antiarrhythmics, antihypertensives, vascular headache suppressants (Calcium channel blockers) Antihypertensives, diuretics PO, PO-ER, IV Dizziness, SOB, CP, cough Arrhythmias, HF, Stevens-Johnson Syndrome. Decreases contractility/conductivity, demand for O2 PO, IV, IM Hypokalemia, dizziness, h/a, weakness, ER Lowering of BP in HTN Pts and diuresis with mobilization of edema PO Supine HTN, urinary urge/retention/freq uency, dysuria, piloerection, pruritus, paresthesia Increase in vascular tone and BP Hydrochloroth iazide (Thiazide diuretics) Midodrine (Proamatine) Alpha-adrenergic agonist Monitor BP, HR, ECG during prolonged therapy. Monitor intake/output. Assess for signs of HF be monitor for toxicity. (pts receiving digoxin concurrently with nifedipine should have routine tests of serum digoxin levels) May cause StevensJohnson syndrome-assess for rash. LABS: monitor serum potassium periodically. Monitor serum K+, hypokalemia increases risk of arrhythmias. Monitor renal and hepatic functions. May cause increase hepatic enzymes. May cause positive ANA and direct Coombs’ Test results Monitor BP, HR, ECG (verapamil may cause prolonged PR interval) Monitor intake and output. Assess for signs of HF. Assess for rash periodically during therapy. Monitor BP, intake, output, and daily wt and assess feet, legs, and sacral area for edema daily. Monitor BP. LABS: Monitor electrolytes (especially potassium), blood glucose, BUN, serum creatinine, and uric acid levels before and periodically during therapy. May cause ↑ serum and urine glucose in diabetic patients. May cause ↑ serum bilirubin, calcium, creatinine, and uric acid, and ↓ serum magnesium, potassium, sodium, and urinary calcium concentrations. May cause ↑ serum cholesterol, low-density lipoprotein, and triglyceride concentrations. Monitor supine and sitting BP prior to and during therapy. Assess pattern of urinary output prior to and during treatment for incontinence. LABS: Monitor renal and hepatic function prior to and periodically during therapy. Atropine Clonidine (Catapres) Antiarrhythmics IV, IM subcut Tachycardia, blurred vision, dry mouth, urinary hesitancy, drowsiness Acts on smooth muscle of heart and increase cardiac rate by inhibiting acetylcholine Assess vital signs and ECG tracings frequently during IV drug therapy. Monitor intake/output. Toxicity: Physostigmine is the antidote. Centrally acting alpha agonist Anti-hypertensive PO, transdermal, epidural Dry mouth, drowsiness, withdrawal phenomenon Deceased BP and pain. Improvement in ADHD symptoms HTN: monitor BP, HR and Intake/ output. Monitor pain. Monitor for opioid withdrawal. Assess for ADHD. LABS: May cause transient ↑ in blood glucose levels. May cause ↓ urinary catecholamine and vanillylmandelic acid (VMA) concentrations; these may ↑ on abrupt withdrawal. May cause weakly positive Coombs' test result HMG-CoA Reductase Inhibitor PO Rash, abd. Cramps, constipation, diarrhea, flatus, heartburn. Rhabdomyolysis, hypersensitivity reactions. Lowers total LDL and triglycerides. Slight increase HDL. Reducing the risk of myocardial infarction and stroke. Slows progression of coronary atherosclerosis. PO, PO-ER Rash, abd. Cramps, constipation, diarrhea, flatus, heartburn. Rhabdomyolysis, Lowering total LDL and triglycerides. Slight increase HDL. Reducing the risk of myocardial infarction and stroke. Slows progression of coronary atherosclerosis. Obtain a diet hx. LABS: monitor liver function f symptoms of serious liver injury, hyperbilirubinemia, or jaundice occurs discontinue atorvastatin and do not restart. May also cause ↑ alkaline phosphatase and bilirubin levels. If patient develops muscle tenderness during therapy, monitor CPK levels. If CPK levels are >10 times the upper limit of normal or myopathy occurs, therapy should be discontinued. Obtain a diet hx. LABS: monitor liver function f symptoms of serious liver injury, hyperbilirubinemia, or jaundice occurs discontinue atorvastatin and do not restart. May also cause ↑ alkaline phosphatase and bilirubin levels. If patient develops muscle tenderness during therapy, monitor CPK levels. If CPK levels are >10 times the upper limit of normal or myopathy occurs, therapy should be discontinued. Statins Atorvastatin (Lipitor) Lipid lowering agent Lovastatin (Mevacor) HMG-CoA Reductase Inhibitor Lipid lowering agent Pravastatin (Pravachol) HMG-CoA Reductase Inhibitor Lipid lowering agent PO Hard on liver Rhabdomyolysis. Abd cramps, constipation, diarrhea, flatus, heartburn Lowering total LDL and triglycerides. Slight increase HDL. Reducing the risk of myocardial infarction and stroke. Slows progression of coronary atherosclerosis. Simvastatin (Zocor) HMG-CoA Reductase Inhibitor Lipid lowering agent PO Hard on liver Rhabdomyolysis. Abd cramps, constipation, diarrhea, flatus, heartburn Used for hypercholesterolemia, prevention of primary/secondary cardiac events Get LFT regularly checked Give medication in evening Obtain a diet hx. LABS: monitor liver function f symptoms of serious liver injury, hyperbilirubinemia, or jaundice occurs discontinue atorvastatin and do not restart. May also cause ↑ alkaline phosphatase and bilirubin levels. If patient develops muscle tenderness during therapy, monitor CPK levels. If CPK levels are >10 times the upper limit of normal or myopathy occurs, therapy should be discontinued. Obtain a diet hx. LABS: monitor liver function f symptoms of serious liver injury, hyperbilirubinemia, or jaundice occurs discontinue atorvastatin and do not restart. May also cause ↑ alkaline phosphatase and bilirubin levels. If patient develops muscle tenderness during therapy, monitor CPK levels. If CPK levels are >10 times the upper limit of normal or myopathy occurs, therapy should be discontinued. Diuretics Spironolactone (Aldactone) Potassium-sparing Diuretic PO Hyperkalemia, drowsiness, dizziness, lightheadedness, stomach upset, diarrhea, nausea, vomiting, or HA Used for CHF, cirrhosis, renal disease, hypertension with edema Monitor BP, intake, and output, assess pt for development of hyperkalemia. Assess for skin rash LABS: Evaluate K+, monitor BUN, serum creatinine, and electrolytes. May cause increase serum magnesium, uric acid, BUN, creatinine, potassium, plasma renin, and urinary calcium excretion. May cause lowering sodium levels. Discontinue potassium-sparing diuretics 3 days prior to a glucose tolerance test because of risk of severe hyperkalemia. May cause false ↑ of plasma cortisol concentrations. Spironolactone should be withdrawn 4–7 days before test Lasix (Furosemide) Diuretics, loop diuretics PO, IV, IM Dehydration, hypocalcemia, hypochloremia, Used for CHF, cirrhosis, renal disease, hypertension with edema Monitor BP, intake, and output, assess pt for development of hyperkalemia. Assess for skin rash hypokalemia, hypomagnesemia, hyponatremia, hypovolemia, metabolic alkalosis Torsemide (Demadex) Antihypertensives Loop diuretic Bumex (Bumetanide) Loop diuretic PO Dehydration, Used for CHF, cirrhosis, hypochloremia, renal disease, hypertension hypokalemia, Monitor I/O’s hypomagnesemia, hyponatremia, hypovolemia, metabolic alkalosis Electrolyte changes, hypotension, weight change, I&O imbalance, dehydration, hyperglycemia Used for edema due to heart failure, hepatic disease, or renal impairment. LABS: Monitor electrolytes, renal and hepatic function, serum glucose, and uric acid levels before and periodically throughout therapy. Commonly ↓ serum potassium. May cause ↓ serum sodium, calcium, and magnesium concentrations. May also cause ↑ BUN, serum glucose, serum creatinine, and uric acid levels. Monitor BP, intake, and output, assess pt for development of hyperkalemia. Assess for skin rash LABS: Monitor electrolytes, renal and hepatic function, serum glucose, and uric acid levels before and periodically throughout therapy. Commonly ↓ serum potassium. May cause ↓ serum sodium, calcium, and magnesium concentrations. May also cause ↑ BUN, serum glucose, serum creatinine, and uric acid levels. Monitor BP, intake, and output, assess pt for development of hyperkalemia. Assess for skin rash LABS: Monitor electrolytes, renal and hepatic function, serum glucose, and uric acid levels before and periodically throughout therapy. Commonly ↓ serum potassium. May cause ↓ serum sodium, calcium, and magnesium concentrations. May also cause ↑ BUN, serum glucose, serum creatinine, and uric acid levels. Vasopressors HIGH ALERT Levophed (Norepinephri ne) Alpha/beta agonist Vasopressor IV Pain, burning, irritation, discoloration, or skin changes, sudden numbness, weakness, slow or uneven HR. Increased BP increased cardiac output. Monitor BP Q2-3min until stabilized and Q5min thereafter. Systolic BP is usually maintained at 80-100 mmHg or 30-40 mm Hg below the previously existing systolic pressure in previously hypertensive pts. ECG should be monitored continuously. CVP, intra-arterial pressure, pulmonary artery diastolic pressure, pulmonary capillary wedge pressure (PCWP), and cardiac output may also be monitored. Monitor urine output and notify health care professional if it decreases to <30 mL/hr. Toxicity: If overdose occurs, discontinue norepinephrine, and administer fluid and Dopamine Inotropic, vasopressors, adrenergics IV Arrhythmias, hypotension Causes vasodilation, increases contractility, SV, and CO High doses: peripheral resistance, increased BP, renal vasoconstriction Best if used through central line*** Dobutamine HIGH alert Inotropic, adrenergics IV HTN, increase HR, Increased cardiac output premature without significantly ventricular increased heart rate. contractions Vasopressin Post pituitary hormone Antidiuretic hormone IM, Subcut. IV HF, myocardial ischemia, limb ischemia Decreased urine output and increased urine osmolarity in diabetes insipidus, increased BP. electrolyte replacement therapy. An alphaadrenergic blocking agent may be administered intravenously to treat hypertension. Monitor BP, HR, pulse pressure, ECG, pulmonary capillary wedge pressure, cardiac output, CVP, and urinary output continuously during administration. Monitor urine output frequently throughout administration. Report decreases in urine output promptly. Palpate peripheral pulses and assess appearance of extremities routinely during dopamine administration. If hypotension occurs, administration rate should be increased. If hypotension continues, more potent vasoconstrictors (norepinephrine) may be administered. Toxicity Overdose: If excessive hypertension occurs, rate of infusion should be decreased or temporarily discontinued until BP is decreased. Monitor BP, heart rate, ECG, pulmonary capillary wedge pressure, cardiac output, CVP, and urinary output continuously during the administration. Palpate peripheral pulses and assess appearance of extremities routinely during dobutamine administration. Notify health care professional if quality of pulse deteriorates or if extremities become cold or mottled. LABS: Monitor potassium concentrations during therapy; may cause hypokalemia. Monitor electrolytes, BUN, creatinine, and prothrombin time weekly during prolonged therapy. Toxicity Overdose: If overdose occurs, reduction or discontinuation of therapy is the only treatment necessary because of the short duration of dobutamine. Monitor BP, HR, and ECG periodically throughout therapy and continuously throughout cardiopulmonary resuscitation. Diabetes Insipidus: Monitor urine osmolality and urine volume frequently to Epinephrine (Adrenalin) Antiasthmatics, bronchodilators, vasopressors, adrenergics Inhaln. Subcut. IM. IV Angina, arrhythmias, hypertension, tachycardia, nervousness, restlessness, tremor Causes vasoconstriction, increases HR, bronchodilation PO, IV Joint pain, dizziness, h/a , N/V, gas, diarrhea Diminished accumulation of acid in the gastric lumen, with lessened acid reflux. Healing of duodenal ulcer and esophagitis. Decrease acid secretion in hypersecretory conditions. determine effects of medication. Assess patient for symptoms of dehydration Weigh patient daily, monitor intake and output, and assess for edema. LABS: Monitor urine specific gravity throughout therapy. Monitor serum electrolyte concentrations periodically during therapy. Toxicity Overdose: Signs and symptoms of water intoxication include confusion, drowsiness, headache, weight gain, difficulty urinating, seizures, and coma. Treatment of overdose includes water restriction and temporary LABS: May cause transient ↓ in serum potassium concentrations with nebulization or at higher than recommended doses. May cause an ↑ in blood glucose and serum lactic acid concentrations. Toxicity Overdose: Symptoms of overdose include persistent agitation, chest pain or discomfort, decreased BP, dizziness, hyperglycemia, hypokalemia, seizures, tachyarrhythmias, persistent trembling, and vomiting. Treatment includes discontinuing adrenergic bronchodilator and other betaadrenergic agonists and symptomatic, supportive therapy. Cardio selective beta blockers are used cautiously because they may induce bronchospasm. Gastrointestinal Protonix (Pantoprazole) Proton Pump Inhibitor Assess patient routinely for epigastric or abdominal pain and for frank or occult blood in stool, emesis, or gastric aspirate. LABS: May cause abnormal liver function tests, including ↑ AST, ALT, alkaline phosphatase, and bilirubin. May cause hypomagnesemia. Monitor serum magnesium prior to and periodically during therapy. Carafate (sucralfate) Antiulcer agents. GI protectants PO Constipation ORAL GI Cocktail (lidocaine/bent yl/Maalox) Protection of ulcers, with subsequent healing. Used as a mixture to numb upper GI tract and decrease acid production while coating the stomach Might be called Green Lizard Decreased GI motility Bentyl (dicyclomine hydrochloride) Anticholinergics PO, IM Palpitations, constipation, urine retention, decreased sweating Maalox (aluminummagnesium hydroxide) Antiulcer agents, antacids PO Constipation, diarrhea Used for heartburn, GI upset, acid indigestion Octreotide (Sandostatin) Somatostatin analogue, growth hormone Subcut, IV, IM, PO Edema, increased sweating, sinusitis, hyperglycemia, abdominal pain, cholelithiasis, diarrhea, nausea, Used for GI bleeds, severe diarrhea, acromegaly, dumping syndrome Assess patient routinely for abdominal pain and frank or occult blood in the stool. Assess for symptoms of irritable bowel syndrome (abdominal cramping, alternating constipation and diarrhea, mucus in stools Assess patient routinely for abdominal distention and auscultate for bowel sounds. If constipation becomes a problem, increasing fluids and adding bulk to the diet may help alleviate the constipating effects of the drug. Monitor intake and output ratios; may cause urinary retention. LABS: Antagonizes effects of pentagastrin and histamine during the gastric acid secretion test. Avoid administration for 24 hr preceding the test. Toxicity Overdose: severe anticholinergic symptoms may be reversed with physostigmine or neostigmine. Antacid: Assess for heartburn and indigestion as well as location, duration, character, and precipitating factors of gastric pain. LABS: Monitor serum phosphate, potassium, and calcium levels periodically during chronic use. May cause ↑ serum calcium and ↓ serum phosphate concentrations. Pepcid (famotidine) Antiulcer agents, histamine h2 antagonists PO, IV vomiting, arthralgia, h/a. Confusion, arrhythmias, agranulocytosis, aplastic anemia Healing and preventions of ulcers. Decreased symptoms of gastroesophageal reflux. Decrease secretion of gastric acid. Assess for epigastric or abdominal pain and frank or occult blood in the stool, emesis, or gastric aspirate LABS: Monitor CBC with differential periodically during therapy. May cause false-negative results in skin tests using allergenic extracts. Histamine antagonists should be discontinued 24 hr prior to the test. May cause an ↑ in serum transaminases and serum creatinine. May cause false-positive results for urine protein; test with sulfosalicylic acid. Psychiatric Ativan (Lorazepam) Anti-anxiety/ Anti-emetic Benzodiazepine PO, Po-XR, IM, IV Dizziness, drowsiness, lethargy, apnea, cardiac arrest Sedation, decreased anxiety, decreased seizures Valium (Diazepam) Antianxiety agents, anticonvulsants, sedative/hypnotics, skeletal muscle relaxants PO, IV, IM Dizziness, drowsiness, lethargy, respiratory depression Relief of anxiety. Sedation. Amnesia. Skeletal muscle relaxation. Decreased seizure activity. Assess risk for addiction, abuse, or misuse prior to administration and periodically during therapy. LABS: Patients on high-dose therapy should receive routine evaluation of renal, hepatic, and hematologic function. Toxicity Overdose: If overdose occurs, flumazenil (Romazicon) is the antidote. Do not use with patients with seizure disorder. May induce seizures. Assess risk for addiction, abuse, or misuse prior to administration and periodically during therapy. LABS: Evaluate hepatic and renal function and CBC periodically during prolonged therapy. May cause ↑ transaminases and alkaline phosphatase. Toxicity Overdose: Flumazenil is an adjunct in the management of toxicity or overdose. (Flumazenil may induce seizures in patients with a history of seizures disorder or who are on tricyclic antidepressants.) Celexa (citalopram) SSRI Anti-depressant PO Lexapro (escitalopram oxalate) SSRI Antidepressants PO antidepressants, Wellbutrin smoking. (bupropion hydrobromide) DETERRENTS. Aminoketone's PO Geodon (ziprasidone) Antipsychotic, mood stabilizers, piperazine derivatives PO, IM Latuda (lurasidone hydrochloride) Dopamineserotonin receptor agonist Anti-psychotic PO Sweating, Antidepressant action. abdominal pain, anorexia, diarrhea, dry mouth, dyspepsia, flatulence, increase saliva, nausea, confusion, drowsiness, insomnia, tremor Diarrhea, nausea, Used for depression, insomnia anxiety, hot flashes Inhibits neuronal reuptake of serotonin Assess for suicidal tendencies. Assess for serotonin syndrome. (Mental changes., agitation, hallucinations, coma, anatomic instability. Tachycardia labile BP. LABS: Monitor electrolytes (potassium and magnesium). In patients at risk for electrolyte imbalances prior to and periodically during therapy. Increased suicidal diminish depression. thoughts, Decrease cravings for dizziness, cigarettes. arrythmia, weight changes, decreased libido Assess mental status and mood changes in all patients. LABS: Monitor, hepatic and renal function closely in patients with kidney or liver impairment. To prevent increased serum and tissue, bupropion on concentration. Make calls false positive urine test for amphetamines Nausea., drowsiness, parkinsonism, akathisia Assess for suicidal tendencies. Assess for serotonin syndrome. (Mental changes [agitation, hallucinations, coma], anatomic instability [Tachycardia, labile BP] Diminished schizophrenic behavior. Reduces symptoms of mania Monitor serum potassium and magnesium prior to and periodically during therapy. Obtain fasting blood glucose and cholesterol levels initially and periodically during therapy. Monitor CBC frequently during initial. May cause leukopenia, neutropenia, or agranulocytosis. Discontinue therapy if this occurs. Monitor serum prolactin prior to and periodically during therapy. May cause ↑ serum prolactin levels Decreases schizophrenic behavior. Decrease depressive episode and bipolar. One disorder. Monitor mood changes assess for suicidal tendencies. LABS: May cause increased serum prolactin levels. May cause increase CPK. Obtain fasting blood glucose and cholesterol levels initially and periodically during therapy. Monitor CBC frequently during initial therapy. Assess degree of anxiety. LABS: Monitor CBC. And liver and renal function periodically during long term therapy period may cause decrease Hematic crit and neutropenia. Toxicity: Flumazenil Is the antidote for alprazolam toxicity or overdose. Monitor closely for notable changes in behavior that could indicate the emergence or worsening of suicidal thoughts. LABS: Patients on prolonged therapy should have CBC and liver function tests. May cause an increase in serum bilirubin, AST, and ALT. May cause decrease thyroidal uptake of 123 I, and 131 I. Toxicity: Therapeutic serum concentrations are 20- 80 mg/ml. Flumazenil Antagonist, clonazepam toxicity or overdose. Xanax (Alprazolam) anti-anxiety agents., PO benzodiazepine, Dizziness, drowsiness, lethargy. Used in anxiety, panic disorders Potentiates effects of GABA to depress the CNS Klonopin (clonazepam) Anticonvulsants, benzodiazepines PO Amnesia, confusion, palpitations, cough, hair loss Prevention of seizures. Decrease manifestation of panic disorder. PO, IV, IM Dizziness, drowsiness, lightheadedness, anorexia, GI upset, nausea Used for skeletal muscle relaxation Assess patient for pain, muscles stiffness, and range of motion. Monitor HR and BP. LABS: Monitor renal function periodically during prolonged parenteral therapy (>3 days), because polyethylene glycol 300 vehicle is nephrotoxic. May cause falsely increased urinary 5hydroxyindoleacetic acid (5-HIAA) and vanillylmandelic acid (VMA) determinations. PO, Extended release Dizziness, sleepiness, confusion, dry mouth, palpitations Dizziness, drowsiness, lethargy, respiratory depression Reduction in muscle spasm and hyperactivity without loss of function Assess patient for pain, muscle stiffness, and range of motion before and periodically throughout therapy. Assess for serotonin syndrome Relief of anxiety. Sedation. Amnesia. Skeletal muscle relaxation. Decreased seizure activity. Assess risk for addiction, abuse, or misuse prior to administration and periodically during therapy. LABS: Evaluate hepatic and renal function and CBC periodically during prolonged therapy. May cause ↑ transaminases and alkaline phosphatase. Toxicity Overdose: Muscle Relaxers Robaxin (Methocarbam ol) Skeletal muscle relaxants Skeletal muscle Flexeril (Cyclobenzapri relaxants ne) Valium (Diazepam) Benzodiazepine PO, IM, IV Ativan (Lorazepam) Anti-anxiety/ Anti-emetic Benzodiazepine PO, Po-XR, IM, IV Dizziness, drowsiness, lethargy, apnea, cardiac arrest Produces muscle relaxation, has antianxiety, anti-emetic, and anticonvulsant. properties Soma Skeletal muscle relaxants PO Dizziness, drowsiness Skeletal muscle relaxation Flumazenil is an adjunct in the management of toxicity or overdose. (Flumazenil may induce seizures in patients with a history of seizures disorder or who are on tricyclic antidepressants.) Assess risk for addiction, abuse, or misuse prior to administration and periodically during therapy. LABS: Patients on high-dose therapy should receive routine evaluation of renal, hepatic, and hematologic function. Toxicity Overdose: If overdose occurs, flumazenil (Romazicon) is the antidote. Do not use with patients with seizure disorder. May induce seizures. Assess patient for pain, muscle stiffness, and range of motion before and periodically during therapy. Observe for idiosyncratic symptoms (extreme weakness, quadriplegia, dizziness, ataxia, dysarthria, visual disturbances, agitation, euphoria, confusion, disorientation); may appear within minutes or hours of first dose. Usually subsides over several hours. Blood Thinner/Anti-platelet Heparin Anti-coagulant Lovenox (Enoxaparin) HIGH Alert antithrombotic low molecular weight heparins Subcut, IV BLEEDING, HEP ARIN-INDUCED THROMBOCYTO PENIA (HIT) (WITH OR WITHOUT THROMBOSIS), a nemia Prevention of thrombus formation. Prevention of extension of existing thrombi. Bruising, bleeding, Prevention of thrombus thrombocytopenia, formation angioedema Assess for signs of bleeding and hemorrhage (bleeding gums; nosebleed; unusual bruising; black, tarry stools; hematuria; fall in hematocrit or BP; guaiacpositive stools). Notify health care professional if these occur. Monitor activated partial thromboplastin time (aPTT) and hematocrit Toxicity Overdose: Protamine sulfate is the antidote. Due to short half-life, overdose can often be treated by withdrawing the drug. Assess for signs of bleeding and hemorrhage (bleeding gums; nosebleed; unusual bruising; black, tarry stools; hematuria; fall in hematocrit or BP; guaiacpositive stools). Notify health care professional if these occur. Monitor CBC, platelet count, and stools for occult blood periodically during therapy. If thrombocytopenia occurs, monitor closely. If hematocrit decreases unexpectedly, assess patient for potential bleeding sites. Toxicity Overdose: For overdose, protamine sulfate 1 mg for each mg of enoxaparin should be administered by slow IV injection. Aspirin (acetylsalicylic acid, ASA) Antiplatelet agents Antipyretics Nonopioid analgesics PO Dyspepsia, epigastric distress, nausea, GI bleeding Pain, fever, angina, stroke, CABG. Warfarin (Coumadin) HIGH Alert Anti-coagulant PO Calciphylaxis, bleeding Prevention of thromboembolic events Eliquis (Apixaban) Anti-coagulant, Factor Xa inhibitor PO Bleeding, anaphylaxis Prevents thrombin generation. Patients who have asthma, allergies, and nasal polyps or who are allergic to tartrazine are at an increased risk for developing hypersensitivity reactions. Monitor for signs and symptoms of DRESS (fever, rash, lymphadenopathy, facial swelling) periodically during therapy. Discontinue therapy if symptoms occur Toxicity Overdose: Monitor for the onset of tinnitus, headache, hyperventilation, agitation, mental confusion, lethargy, diarrhea, and sweating. If these symptoms appear, withhold medication and notify health care professional immediately. Assess for signs of bleeding and hemorrhage (bleeding gums; nosebleed; unusual bruising; tarry, black stools; hematuria; fall in hematocrit or BP; guaiacpositive stools, urine, or nasogastric aspirate). LABS: Monitor PT, INR Toxicity Overdose: Withholding 1 or more doses of warfarin is usually sufficient if INR is excessively elevated or if minor bleeding occurs. If overdose occurs or anticoagulation needs to be immediately reversed, the antidote is vitamin K (phytonadione, Aquamephyton). Administration of whole blood or plasma also may be required in severe bleeding because of the delayed onset of vitamin K. Assess patient for symptoms of stroke, DVT, PE, bleeding, or peripheral vascular disease periodically during therapy. Toxicity Overdose: To prevent stroke with a.fib, DVT prevention postsurgery Plavix (Clopidogrel) Antiplatelet agents Platelet aggregation inhibitors PO GI bleeding, bleeding, neutropenia, thrombotic thrombocytopenic purpura, ACUTE GENERALIZED EXANTHEMATOUS PUSTULOSIS, DRU G RASH WITH EOSINOPHILIA AND SYSTEMIC SYMPTOMS, STEVE NS-JOHNSON SYNDROME, TOXIC EPIDERMAL NECROLYSIS Reduction in risk of MI and stroke Arixtra (fondaparinux sodium) Anticoagulants, active factor x inhibitors Subcut Bleeding, increased liver levels Interruption of the coagulation cascade resulting in inhibition of thrombus formation. Prevention of thrombus formation decreases the risk of pulmonary emboli Antidote is andexanet alfa. Effects persist for at least 24 hrs after last dose. Oral activated charcoal decreases apixaban absorption, lowering plasma concentrations. Other agents and hemodialysis do not have a significant effect. Assess patient for symptoms of stroke, peripheral vascular disease, or MI periodically during therapy. Monitor patient for signs of thrombotic thrombocytopenic purpura LABS: Monitor bleeding time during therapy. Prolonged bleeding time, which is time- and dose-dependent, is expected. Monitor CBC with differential and platelet count periodically during therapy. Neutropenia and thrombocytopenia may rarely occur. May cause ↑ serum bilirubin, hepatic enzymes, total cholesterol, nonprotein nitrogen, and uric acid concentrations Assess for signs of bleeding and hemorrhage. Monitor platelet count closely; may cause thrombocytopenia. If platelet count is <100,000/mm3 , discontinue fondaparinux. Fondaparinux is not accurately measured by prothrombin time (PT), activated thromboplastin time (aPTT), or international standards of heparin or lowmolecular-weight heparins. If unexpected changes in coagulation parameters or major bleeding occurs, discontinue fondaparinux. Monitor CBC, serum creatinine levels, and stool occult blood tests routinely during therapy. May cause asymptomatic ↑ in AST and ALT. Elevations are fully reversible and not associated with ↑ in bilirubin. May cause ↑ aPTT temporally associated with bleeding with or without concomitant administration of other anticoagulants and thrombocytopenia with thrombosis similar to heparin-induced thrombocytopenia, with or without exposure to heparin or lowmolecular-weight heparins. Assess for symptoms of stroke or peripheral vascular disease periodically during therapy. Assess for symptoms of bleeding and blood loss; may be fatal. If reversal of anticoagulant effect is required, may use idarucizumab. LABS: Use aPTT or ecarin clotting time (ECT), not INR, to assess anticoagulant activity, if needed. Monitor renal function prior to and periodically during therapy. Patients with renal impairment may require dose reduction or discontinuation. Assess for bleeding Lab Test Considerations: May cause ↑ serum AST, ALT, total bilirubin, and GGT levels. Monitor renal function periodically during therapy. Toxicity Overdose: Antidote is andexanet alfa; effects persist for at least 24 hr after last dose. Other agents and hemodialysis do not have a significant effect. Consider using prothrombin complex concentrate (PCC) or Factor VIIa. Pradaxa (dabigatran) Anticoagulants PO Thrombi inhibitions Bleeding, abd pain Reduced risk of thrombotic sequelae (stroke and systemic embolism) in nonvalvular AF. Reduced risk of recurrent PE and DVT. Resolution of DVT and PE. Xarelto (Rivaroxaban) Anticoagulants Antithrombotics Factor xa inhibitors PO Bleeding Used to prevent DVT, PE, emboli from a.fib. PO, IV Torsades de pointes, stevensjohnson syndrome, hepatotoxicity Prevention of fungal infection Lab Test Considerations: Monitor BUN and serum creatinine before and periodically during therapy; patients with renal dysfunction will require dose adjustment. Monitor liver function tests before and periodically during therapy. May cause ↑ AST, ALT, serum alkaline phosphate, and bilirubin concentrations. Diarrhea, nausea stomach pain Binds to fungal cell wall altering permeability. Inspect oral mucous membranes before and frequently during therapy. Increased irritation of mucous membranes may indicate need to discontinue medication Anti-fungal Diflucan (Fluconazole) Antifungal Nystatin Anti-fungal PO Used for intestinal candidiasis, thrush, mycotic infections Thyroid Thyroid hormone Synthroid (Levothyroxine ) PO, IV Weight gain/loss, h/a, vomiting, diarrhea, fever Replacement in hypothyroidism to restore normal hormonal balance. Suppression of thyroid cancer. Assess apical pulse and BP prior to and periodically during therapy. Assess for tachyarrhythmias and chest pain Lab Test Considerations: Monitor thyroid function studies prior to and during therapy. Monitor thyroidstimulating hormone (TSH) serum levels after start. Monitor blood and urine glucose in diabetic patients. Insulin or oral hypoglycemic dose may need to be increased. Toxicity Overdose: Overdose is manifested as hyperthyroidism (tachycardia, chest pain, nervousness, insomnia, diaphoresis, tremors, weight loss). Usual treatment is to withhold dose for 2–6 days then resume at a lower dose. Acute overdose is treated by induction of emesis or gastric lavage, followed by activated charcoal. Sympathetic overstimulation may be controlled by antiadrenergic drugs (beta blockers), such as propranolol. Oxygen and supportive measures to control symptoms are also used. Cardiac arrest, phlebitis at IV site, suicidal thoughts, apnea, laryngospasm, respiratory depression Short-term sedation. Postoperative amnesia. Termination of seizure activity. Assess level of sedation and level of consciousness throughout and for 2–6 hr following administration. Monitor BP, pulse, and respiration continuously during administration, especially if co-administering opioid analgesics. Oxygen and resuscitative equipment should be immediately available during IV administration. Assess risk for addiction, abuse, or misuse before starting and periodically during therapy. Toxicity Overdose: Anti-Convulsant Versed (Midazolam) Benzodiazepine IN, IM, IV Keppra (levetiracetam) Anticonvulsants IV, PO Ativan (Lorazepam) Anti-anxiety/ Anti-emetic Benzodiazepine PO, IV, IM Dilantin (Phenytoin) Antiarrhythmics, anticonvulsants PO, PO-ER, IV Aggression, agitation, anger, anxiety, apathy, depersonalization, depression, dizziness, drowsiness, fatigue, hostility, irritability, personality disorder, psychosis, weakness Dizziness, drowsiness, lethargy, apnea, cardiac arrest Inhibits simultaneous neuronal firing. Used epilepsy, tonic-clonic seizures Gingival hyperplasia Bradycardia GI upset, headache, insomnia Diminished seizures activity. Termination of ventricular arrhythmias. Calms and sedates by causing muscle relaxation If overdose occurs, monitor pulse, respiration, and BP continuously. Maintain patent airway and assist ventilation as needed. If hypotension occurs, treatment includes IV fluids, repositioning, and vasopressors. The effects of midazolam can be reversed with flumazenil (Romazicon). Monitor mood changes. Assess for suicidal tendencies, especially during early therapy. Restrict amount of drug available to patient. Assess for rash periodically during therapy. May cause Stevens-Johnson syndrome. Discontinue therapy if severe or if accompanied with fever, general malaise, fatigue, muscle or joint aches, blisters, oral lesions, conjunctivitis, hepatitis, and/or eosinophilia. LABS: May cause ↓ RBC and WBC and abnormal liver function tests. Assess risk for addiction, abuse, or misuse prior to administration and periodically during therapy. LABS: Patients on high-dose therapy should receive routine evaluation of renal, hepatic, and hematologic function. Toxicity Overdose: If overdose occurs, flumazenil (Romazicon) is the antidote. Do not use with patients with seizure disorder. May induce seizures. Monitor closely for notable changes in behavior that could indicate the emergence or worsening of suicidal thoughts or behavior or depression. LABS: Monitor CBC, serum calcium, albumin, and hepatic function tests prior to and monthly for the first several mo, then periodically during therapy. May cause ↑ serum alkaline phosphatase, GGT, and glucose levels. Monitor serum folate concentrations periodically during prolonged therapy. Toxicity Overdose: Tegretol (Carbamazepi ne) Anticonvulsant, mood stabilizers Antiepileptic Valproic Acid (Depakene/Dep akote) Monitor serum phenytoin levels routinely. Therapeutic blood levels are 10–20 mcg/mL (8–15 mcg/mL in neonates) in patients with normal serum albumin and renal function. In patients with altered protein binding (neonates, patients with renal failure, hypoalbuminemia, acute trauma), free phenytoin serum concentrations should be monitored. Therapeutic serum free phenytoin levels are 1–2 mcg/mL. Progressive signs and symptoms of phenytoin toxicity include nystagmus, ataxia, confusion, nausea, slurred speech, and dizziness. Monitor closely for changes in behavior that could indicate the emergence or worsening of suicidal thoughts or behavior or depression LABS: Monitor CBC, including platelet count, reticulocyte count, and serum iron, at baseline, weekly during the first 2 mo, and yearly thereafter for evidence of potentially fatal blood cell abnormalities. Discontinue therapy if bone marrow depression occurs. PO, PO-ER Ataxia, drowsiness, hepatotoxicity, pancreatitis Used for tonic-clonic and partial seizures. Reduces synaptic reaction. Frequent level checks PO, IV Hepatotoxicity, pancreatitis, suicidal thoughts Prevention of seizures. Relief of pain in trigeminal neuralgia, Decreased mania. Monitor closely for changes in behavior that could indicate the emergence or worsening of suicidal thoughts or behavior or depression LABS: Monitor CBC, including platelet count, reticulocyte count, and serum iron, at baseline, weekly during the first 2 mo, and yearly thereafter for evidence of potentially fatal blood cell abnormalities. Discontinue therapy if bone marrow depression occurs. Toxicity: Serum blood levels should be routinely monitored during therapy. Therapeutic levels range from 4–12 mcg/mL. Arrhythmias, hypocalcemia, weakened/absent Treatment/prevention of hypomagnesemia. Prevention and treatment of Hypomagnesemia/Anticonvulsant: Monitor pulse, BP, respirations, and ECG frequently during administration of Electrolyte Replacement Magnesium (Magnesium sulfate) Minerals electrolytes IV, PO deep tendon reflexes High Alert seizures associated with severe eclampsia or preeclampsia. Potassium (Potassium chloride) Electrolyte replacement PO Arrhythmias, abdominal pain, diarrhea, flatulence, nausea, vomiting. Treatment of hypokalemia Do NOT crush PO Hang IV with fluids to decrease burning Calcium (Calcium gluconate, chloride) Mineral and electrolyte replacements/ supplements IV, PO Arrhythmias, cardiac arrest, constipation, phlebitis Replacement of calcium in deficiency states. parenteral magnesium sulfate. Respirations should be at least 16/min before each dose. LABS: Monitor serum magnesium levels and renal function periodically during administration of parenteral magnesium sulfate. Assess for signs and symptoms of hypokalemia (weakness, fatigue, U wave on ECG, arrhythmias, polyuria, polydipsia) and hyperkalemia LABS: K+, monitor renal function, serum bicarbonate, and pH Toxicity: Symptoms of toxicity are those of hyperkalemia (slow, irregular heartbeat; fatigue; muscle weakness; paresthesia; confusion; dyspnea; peaked T waves; depressed ST segments; prolonged QT segments; widened QRS complexes; loss of P waves; and cardiac arrhythmias) Treatment includes discontinuation of potassium, administration of sodium bicarbonate to correct acidosis, dextrose and insulin to facilitate passage of potassium into cells, calcium salts to reverse ECG effects (in patients who are not receiving digoxin), sodium polystyrene used as an exchange resin, and/or dialysis for patient with impaired renal function. Observe patient closely for symptoms of hypocalcemia (paresthesia, muscle twitching, laryngospasm, colic, cardiac arrhythmias, Chvostek's or Trousseau's sign). Monitor BP, pulse, and ECG frequently during parenteral therapy. May cause vasodilation with resulting hypotension, bradycardia, arrhythmias, and cardiac arrest. Transient increases in BP may occur during IV administration, especially in geriatric patients or in patients with hypertension. LABS: Monitor serum calcium or ionized calcium, chloride, sodium, potassium, magnesium, albumin, and parathyroid hormone (PTH) concentrations before and periodically during therapy for treatment of hypocalcemia Toxicity: Assess patient for nausea, vomiting, anorexia, thirst, severe constipation, paralytic ileus, and bradycardia. Contact health care professional immediately if these signs of hypercalcemia occur Allergic Reaction Antihistamine Benadryl (diphenhydra mine) PO, IM, IV Anorexia, dry mouth, drowsiness Epinephrine bronchodilators, vasopressors, adrenergics IV, IM Angina, arrhythmias, HTN, tachycardia, nervousness, restlessness, tremor Pepcid (famotidine) Histamine h2 antagonists PO, IV Constipation, confusion, diarrhea, dizziness/HA, Arrhythmias Decreased symptoms of histamine excess (sneezing, rhinorrhea, nasal and ocular pruritus, ocular tearing and redness, urticaria). Relief of acute dystonic reactions. Prevention of motion sickness. Suppression of cough. Used for anaphylaxis, hypotension r/t septic shock, cardiac resuscitation, asthma Healing and prevention of ulcers. Decreased symptoms of gastroesophageal reflux. Decreased secretion of gastric acid. LABS: May ↓ skin response to allergy tests. Discontinue 4 days before skin testing LABS: May cause transient ↓ in serum potassium concentrations with nebulization or at higher than recommended doses. May cause an ↑ in blood glucose and serum lactic acid concentrations. Toxicity: Symptoms of overdose include persistent agitation, chest pain or discomfort, decreased BP, dizziness, hyperglycemia, hypokalemia, seizures, tachyarrhythmias, persistent trembling, and vomiting. Treatment includes discontinuing adrenergic bronchodilator and other betaadrenergic agonists and symptomatic, supportive therapy. Cardio selective beta blockers are used cautiously because they may induce bronchospasm. Assess for epigastric or abdominal pain and frank or occult blood in the stool, emesis, or gastric aspirate. LABS: Monitor CBC with differential periodically during therapy. Insulins/Blood Sugar Control Rapid Acting Insulin (Humalog, Novolog) Insulin Short Acting Insulin (Humulin R) Insulin Intermediate Acting Insulin (NPH, Detemir) Insulin SC, IV Hypoglycemia SC, IV Control of hyperglycemia in diabetic patients. Assess for symptoms of hypoglycemia (anxiety; restlessness; tingling in hands, feet, lips, or tongue; chills; cold sweats; confusion; cool, pale skin; difficulty in concentration; drowsiness; nightmares or trouble sleeping; excessive hunger; headache; irritability; nausea; nervousness; tachycardia; tremor; weakness; unsteady gait)and hyperglycemia (confusion, drowsiness; flushed, dry skin; fruit-like breath odor; rapid, deep breathing, polyuria; loss of appetite; unusual thirst) periodically during therapy. Control of hyperglycemia in diabetic patients. Lab Test Considerations: Monitor blood glucose every 6 hr during therapy, more frequently in ketoacidosis and times of stress. A1C may be monitored every 3–6 mo to determine effectiveness. Monitor serum potassium in patients at risk for hypokalemia (those using potassiumlowering agents, those receiving IV insulin) periodically during therapy. Control of hyperglycemia in diabetic patients. Toxicity Overdose: Overdose is manifested by symptoms of hypoglycemia. Mild hypoglycemia may be treated by ingestion of oral glucose. Severe hypoglycemia is a life-threatening emergency; treatment consists of IV glucose, glucagon, or epinephrine Control of hyperglycemia in diabetic patients. “same” Maintenance of blood glucose When combined with oral sulfonylureas, observe for signs and symptoms of hypoglycemic reactions (abdominal pain, sweating, hunger, weakness, dizziness, headache, tremor, tachycardia, anxiety). Hypoglycemia SC, IV Hypoglycemia Hypoglycemia Long-Acting Insulin (Lantus) Insulin Metformin Biguanide Antidiabetics SC, IV Hypoglycemia PO Lactic acidosis, abd bloating, diarrhea, nausea, vomiting Patients who have been well controlled on metformin who develop illness or laboratory abnormalities should be assessed for ketoacidosis or lactic acidosis. Assess serum electrolytes, ketones, glucose, and, if indicated, blood pH, lactate, pyruvate, and metformin levels. If either form of acidosis is present, discontinue metformin immediately and treat acidosis. Patients with severe renal impairment are at greatest risk for lactic acidosis. Glipizide Sulfonylureas PO D50 Dextrose and water IV Glucagon Hormone pancreatic IV, SC, IM Hypoglycemia Lowering of blood sugar in diabetic patients. “Observe for signs and symptoms of hypoglycemic reactions (sweating, hunger, weakness, dizziness, tremor, tachycardia, anxiety). Patients on concurrent betablocker therapy may have very subtle signs and symptoms of hypoglycemia. Assess patient for allergy to sulfonamides. Lab Test Considerations: Monitor serum glucose and glycosylated hemoglobin (HbA1C ) periodically during therapy to evaluate effectiveness of treatment. Monitor CBC periodically during therapy. Report ↓ in blood counts promptly. May cause an ↑ in AST, LDH, BUN, and serum creatinine. Toxicity Overdose: Overdose is manifested by symptoms of hypoglycemia. Mild hypoglycemia may be treated with administration of oral glucose. Treat severe hypoglycemia with IV D50W followed by continuous IV infusion of more dilute dextrose solution at a rate sufficient to keep serum glucose at approximately 100 mg/dL. “same” Epistaxis, eye redness, itchy eyes, itchy throat, Increase in blood glucose. Assess for signs of hypoglycemia (sweating, hunger, weakness, headache, dizziness, tremor, irritability, tachycardia, nasal congestion, nasal discomfort, nasal itching, nausea, vomiting. Relaxation of GI musculature, facilitating radiographic examination. anxiety) prior to and periodically during therapy.