Benedict's Test: Objectives, Principle, Procedure, Results
advertisement

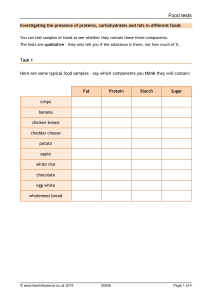
Benedict’s Test- Objectives, Principle, Procedure, Results Table of Contents Introduction Objectives of Benedict’s Test Principle of Benedict’s Test The procedure of Benedict’s Test Observation (Results) of Benedict’s Test References Introduction There are generally two types of sugar namely reducing and non-reducing sugar based on their reducing property. All the monosaccharides are reducing sugars as they have free anomeric carbon in their structure (free aldehyde group or a free ketone group) that can reduce cupric salt. However, in the disaccharides, the monosaccharide unit is linked together by glycosidic linkage formed between the OH hydroxyl group of one and the anomeric carbon of others. Some disaccharides cannot reduce cupric salt due to no availability of free anomeric carbon in their structure and hence they are termed as non-reducing sugars. Monosaccharides can be detected based on their oxidation in alkaline solution by Cu⁺⁺ or Ag⁺⁺ or ferricyanide, the mild oxidizing agent. The reducing property can be determined and demonstrated using Benedict’s solution. Benedict’s test is used to test for simple carbohydrates. Benedict’s test identifies reducing sugars (monosaccharides and some disaccharides), which have free ketone or aldehyde functional groups. Benedict’s solution can be used to test for the presence of glucose in urine. Some sugars such as glucose are called reducing sugars because they are capable of transferring hydrogens (electrons) to other compounds, a process called reduction. When reducing sugars are mixed with Benedict’s reagent and heated, a reduction reaction causes Benedict’s reagent to change color. The color varies from green to dark red (brick) or rusty-brown, depending on the amount of and type of sugar. Benedict’s quantitative reagent contains potassium thiocyanate and is used to determine how much reducing sugar is present. This solution forms a copper thiocyanate precipitate which is white and can be used in a titration. The titration should be repeated with 1% glucose solution instead of the sample for calibration Objectives of Benedict’s Test To determine the presence or absence of reducing sugar in the solution. To determine the glucose concentration in the solution quantitatively. Principle of Benedict’s Test Benedict’s test is performed by heating the reducing sugar solution with Benedict‘s reagent. The presence of the alkaline sodium carbonate converts the sugar into a strong reducing agent called enediols. During the reaction, enediols decrease the cupric particles (Cu2+) present in Benedict’s reagent to cuprous particles (Cu+) which appear as red copper oxide (Cu2O) which is insoluble in water. When Benedict’s reagent solution and reducing sugars are heated together, the solution changes its color to orange-red/ brick red precipitate. The red-colored cuprous oxide is insoluble in water and hence, separates out from the solution. When the concentration of the reducing sugar is high in the solution, then the color becomes more intense (reddish) and the volume of the precipitate increases in the test tube making it clearly visible. image source: chemistrylearner.com Benedict’s solution is a deep-blue alkaline chemical reagent used to test for the presence of the aldehyde functional group -CHO which consists of copper sulfate pentahydrate (CuSO4. 5H2O), sodium carbonate (Na2CO3), sodium citrate (Na3C6H5O7), and distilled water. Sodium carbonate renders alkaline conditions which are required for the redox reaction, while sodium citrate is a complexing agent which complexes with the copper (II) ions to avoid degradation into copper (I) ions during storage. Complex carbohydrates such as starches DO NOT react positively with Benedict’s test unless they are broken down through heating or digestion (try chewing crackers and then doing the test). Table sugar (disaccharide) is a non-reducing sugar and does also not react with the iodine or with the Benedict Reagent. Sugar needs to be decomposed into its components glucose and fructose then the glucose test would be positive but the starch test would still be negative. The procedure of Benedict’s Test 1. Pipette out 2 ml (10 drops) of Benedict’s reagent and placed it in the clean test tube 2. Approximately 1 ml of sample (urine) is added to Benedict’s reagent. 3. The test tube is placed over the boiling water bath for 3-5 minutes (can be heated directly over flame). 4. Observe for color change in the solution of test tubes or precipitate formation. Note : Quantity mentioned in the procedure is tentative depending on the experiment performer Observation (Results) of Benedict’s Test If the color upon boiling is changed into green, then there would be 0.1 to 0.5 percent sugar in solution. If it changes color to yellow, then 0.5 to 1 percent sugar is present. If it changes to orange, then it means that 1 to 1.5 percent sugar is present. If the color changes to red, then 1.5 to 2.0 percent sugar is present. And if the color changes to brick red, it means that more than 2 percent of sugar is present in the solution. image source: chemistrylearner.com Positive Benedict’s Test: Formation of a reddish precipitate within three minutes. Reducing sugars present. Example: Glucose Negative Benedict’s Test: No color change (Remains Blue). Reducing sugars absent. Example: Sucrose. Appearance of solution The concentration of reducing sugar (g%) Interpretation Brick red with heavy precipitate Brownish orange with red precipitate Yellow with precipitate 2% or >2% 1.5% 1% Greenish blue and cloudy 0.5% Greenish blue with yellow precipitate 0.25% Green with no precipitate 0.1% Blue color or cloudy Nil https://youtu.be/XBiv_s0v1yc A large amount of reducing sugar is pr A moderate amount reducing sugar is pr A small amount of reducing sugar is pr Traceable amount o reducing sugar is pr Traceable amount o reducing sugar is pr Traceable amount o reducing sugar is pr No reducing sugar i present References Shrestha B (2002). Practical biochemistry and biotechnology. First edition. 99933665-1-X National Institutes of Health, Testing for Lipids, Proteins and CarbohydratesBenedict’s solution. Fayetteville State University- Biological Molecules: Carbohydrates, Lipids, Proteins. Harper College- Benedict’s Test. Chemistry learner- Benedict’s Test. Northern Kentucky University- Benedict’s Reagent: A Test for Reducing Sugars. KNUST Open Educational Resources, Benedict’s Test – Qualitative Test in Carbohydrates. Amrita Virtual Lab Collaborative Platform- Qualitative Analysis of Carbohydrates. National Institutes of Health, Testing for Lipids, Proteins and CarbohydratesBenedict’s solution. Fayetteville State University- Biological Molecules: Carbohydrates, Lipids, Proteins. Harper College- Benedict’s Test. National Biochemicals Corp.- BENEDICT’S SOLUTION (MB4755). Science Olympiad- Use of Benedict’s Solution. Brilliant Biology Student 2015- Food Tests- Benedict’s Test for Reducing Sugars. BBC Bitesize- Chemistry- Carbohydrates. University of Manitoba- The Molecules of Life: Biochemistry- Carbohydrates. Northern Kentucky University- Benedict’s Reagent: A Test for Reducing Sugars. KNUST Open Educational Resources, Benedict’s Test – Qualitative Test in Carbohydrates. Mark Rothery’s Biology Web Site- Biochemical Tests. All Medical Stuff- Benedict’s test for reducing sugar. Hendrix College- Benedicts Test for Glucose. Info Please- Benedict’s solution. Mystrica- Benedict’s Test. Amrita Virtual Lab Collaborative Platform- Qualitative Analysis of Carbohydrates. Wikipedia. Benedict’s Test : Principle, Reagent Preparation, Procedure and Interpretation https://www.chemistrylearner.com/benedicts-test.html https://www.worldofchemicals.com/557/chemistry-articles/benedicts-reagent-testfor-monosaccharides.html https://diabetestalk.net/blood-sugar/why-can-benedicts-solution-be-used-todistinguish-between-glucose-and-fructose https://answersdrive.com/what-is-a-positive-test-result-for-the-benedict-s-test2674136 https://www.mytutor.co.uk/answers/11705/A-Level/Biology/How-would-you-testfor-the-presence-of-a-non-reducing-sugar/ https://www.answers.com/Q/Why_does_a_brick_red_ precipitate_form_during_the_Benedict’s_test https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-bicarbonate https://in.answers.yahoo.com/question/index?qid=20070823082340AAAwkGd https://en.m.wikipedia.org/wiki/Reducing_sugar https://answers.yahoo.com/question/index?qid=20110918113930AAlMfFU https://www.studymode.com/subjects/lab-report-carbohydrates-proteins-lipids- page1.html https://www.biologyexams4u.com/2012/10/differences-between-reducing-andnon.html https://quizlet.com/72717061/unit-2-practical-biochemistry-flash-cards/ https://quizlet.com/157972611/monosaccharides-flash-cards/ https://quizlet.com/11483276/biology-chapter-2-biological-molecules-flash-cards/ http://wpage.unina.it/petrilli/organic/carbo.htm?html