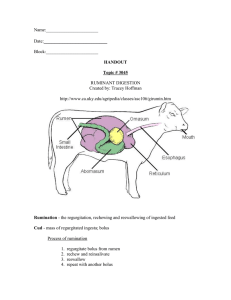
Immunology Letters 162 (2014) 69–76 Contents lists available at ScienceDirect Immunology Letters journal homepage: www.elsevier.com/locate/immlet Review Rumen transfaunation E.J. DePeters a,∗ , L.W. George b a b Department of Animal Science, University of California, Davis, CA, USA School of Veterinary Medicine, University of California, Davis, CA, USA a r t i c l e i n f o Article history: Available online 26 September 2014 Keywords: Rumen transfaunation Rumen fluid Simple indigestion Microorganisms a b s t r a c t The aim of this invited mini-review is to summarize the rumen transfaunation literature. Rumen transfaunation using the cud from a healthy donor animal to treat a sick recipient animal was practiced long before our understanding of rumen microorganisms. Around the mid-1900s, scientists began to explore the benefits of rumen transfaunation and the associated microbial populations. Rumen transfaunation has been used clinically to treat indigestion and to enhance the return of normal rumen function following surgical correction of a left-displaced abomasum. Rumen transfaunation was also used to introduce unique rumen microorganisms into animals that were exposed to toxic compounds in plants. Rumen liquor contains chemical constituents that likely contribute to the beneficial effects of re-establishing a normal reticulo-rumen anaerobic fermentation. Recommendations for collecting rumen fluid, storage and volumes transferred are discussed. Rumen transfaunation is a common practice to treat indigestion on dairy and livestock operations. The support of a healthy microbial community in the digestive tract is also used for humans. Fecal microbiota transplantation has been used to treat digestive disorders in humans. Rumen transfaunation, although not widely studied with respect to mode of action, is an effective, practical, and easy method to treat simple indigestion of ruminants. Published by Elsevier B.V. 1. Introduction The aim of this invited mini-review is to summarize the information available related to rumen transfaunation. Even though the method has been practice for decades [1] and is a common medical practice in food animal medicine to treat simple indigestion of ruminants [2–8], there is a paucity of scientific information to describe its benefits. The ruminant can be considered to be a superorganism because it has a symbiotic relationship of life between the cells of the animal’s body and the rumen microbes. Factors affecting the viability of microorganisms in the reticulo-rumen as well as anywhere along the gastro-intestinal tract of the ruminant impact the host animal. Ruminants are mammals (Class – Mammalia) in the Order Arteriodactyla (even toed, hooved mammals) Suborder Ruminantia. Ruminant, from the Latin word ruminare, means to chew over gain hence the designation of cud chewing. Ruminants have a stomach with four compartments or chambers – reticulum, rumen, omasum, and abomasum (Fig. 1). The reticulum, rumen, and omasum ∗ Corresponding author at: Department of Animal Science, University of California, 1 Shields Avenue, Davis, CA, USA. Tel.: +1 530 752 1263; fax: +1 530 752 0175. E-mail addresses: ejdepeters@ucdavis.edu (E.J. DePeters), lwgeorge@ucdavis.edu (L.W. George). http://dx.doi.org/10.1016/j.imlet.2014.05.009 0165-2478/Published by Elsevier B.V. are lined with non-glandular mucous membranes while the abomasum, the gastric compartment, is lined with glandular mucosa. The abomasum is similar in function to the human stomach. The largest compartment is the rumen, which along with the reticulum, serve as sites of anaerobic fermentation. There are coronary grooves in the rumen creating sacs. There is a cranial groove that separates the reticulum and rumen, and in cattle, sheep, and goats the two compartments are easily distinguished with the reticulum having a honey combed appearance. These compartments are lined with finger like projections called papillae that absorb nutrients (e.g. volatile fatty acids produced by the rumen microbiota). These finger-like projects are nature’s way of increasing the absorptive surface area of the reticulum and rumen. Often ruminant nutritionists refer to these compartments as the reticulo-rumen because together they function in the rumen cycle (coordinated contractions) to support the acts of eructation and rumination. The contractions of the rumen cycle inoculate new food with microorganisms, distribute the end products of digestion for absorption by the mucosa papillae, and pass digesta to the omasum. Eructation is the process by which ruminants release gases from the reticulo-rumen that are produced during anaerobic fermentation. Eructation is a quiet process that involves eructated gas passing up the esophagus and into the trachea and the lungs to be respired. Rumination involves bringing a bolus of digesta up the esophagus (regurgitate) into the mouth where the bolus of digesta (cud) is 70 E.J. DePeters, L.W. George / Immunology Letters 162 (2014) 69–76 Fig. 1. Ruminant stomach. http://biobook.nerinxhs.org/bb/systems/digestion/ 1000px-Abomasum-ia-omaso.svg.png. chewed. The cud is eventually re-swallowed and the process continues with another bolus regurgitated for cud chewing (crushing and grinding of particles by the molars). Cud chewing increases surface area of the feed particles, in particular fibrous material, to enhance microbial digestion. The act of cud chewing also stimulates salvia production, and the buffers present in the saliva help to maintain rumen pH when the bolus is re-swallowed. Digesta leaves the reticulum via the reticulo-omasal orifice. The omasum, with its many leaves or laminae, controls flow of digesta to the abomasum. The abomasum is the gastric, glandular compartment similar to the stomach of nonruminants (human, pig, mouse) with secretion of acid (HCl) and pepsinogen and a pyloric sphincter that regulates flow of digesta from the abomasum to the duodenum. Transfaunation in its current use includes a broad spectrum of microorganisms including bacteria, protozoa, fungi, and archaea that are transferred from rumen of a donor to the rumen of a recipient. In his seminal book on rumen microbiology, Hungate [9] stated that transfaunation of protozoa occurred when protozoa that were left on food by one animal were consumed by another animal. Young animals were faunated by their mothers when she licked them. It is not clear based on our reading of the literature where the term ‘transfaunation’ originated or how it was derived. One dictionary definition of transfaunation is “transfer of symbiotic fauna (usually mutualistic protozoa) from one host to another”. Originally protozoa were defined as unicellular protists. Some ciliated protozoa have the ability to move similar to animals. Protozoa are also eukaryotic while bacteria are prokaryotic. Defaunation of the rumen referred to elimination of protozoa [9]. One dictionary definition of defaunate is “elimination of microscopic fauna, especially protozoa, in the rumen and cecum, with depressing effects on digestion”. Although the meaning of transfaunation is not clearly defined in the literature, in this review we will use the term transfaunation broadly. The discussion of rumen transfaunation will include both microflora and to a limited extent chemical constituents in the rumen contents. 2. Background Brag and Hansen [1] stated rumen transfaunation was used long before research demonstrated the importance of the rumen microorganisms to animal metabolism. These authors reported that the earliest printed reference about transfaunation in Sweden that they found was from 1776 (Hjortberg) that stated “It is common practice, even in the country side, to take the fodder out of the mouth of a sheep or a goat to give it to an animal which does not ruminate.” Subsequent research related to the discovery of the importance of rumen microbial population occurred much later in the 1900s. Research that was conducted at The Ohio Agricultural Experiment Station determined that the cud inoculated rumens of preweaned calves contained bacteria and protozoa as early as 3 weeks of age [10]. Rumens of non-inoculated control calves that were fed milk and alfalfa hay had only bacteria. A similar response in calves was observed when the cud material was obtained from cows grazing pasture [11]. Cud inoculated calves also digested a higher proportion of cellulose and dry matter compared with noninoculated controls [12]. This advantage disappeared later once calves were fed an all forage diet. Presumably this loss of digestion advantage with inoculation was associated with normal rumen development of microflora at weaning. Rumen inoculation was subsequently used as a treatment method to impact calf health. In a field study with a herd experiencing bloody diarrhea and death of preweaned calves, rumen transfaunation improved calf health and survival [13]. Rumen fluid [14] from an alfalfa hay fed steer was transferred into protozoa free sheep that were fed either alfalfa (n = 3) or a high concentrate diet (n = 3). All 24 species of protozoa were established in the rumen of sheep fed alfalfa but only 9 species were established in rumen of sheep fed concentrate. Rumen protozoa play an important role in transfaunation. Rumen protozoa are predominately ciliates of two types: Entodiniomorphid protozoa and Holotrichs [15]. Garry [16] noted that rumen protozoa were sensitive to pH, which supports the lower number of protozoa when sheep were fed a concentrate diet [14]. Most rumen ciliates utilize starch and their numbers increased [15]. Feeding starch that caused a decrease in rumen pH reduced or eliminated rumen protozoa with the larger Holotrichs more sensitive to low pH. However, it is not simply the starch content of the diet that impacts rumen pH and protozoa numbers, but also the type of starch and its rumen availability, the fiber content of the diet, and the physical form of the fiber source, as well as other factors [15,17]. These dietary factors should be considered with respect to the rumen environment of both the donor and recipient animals when rumen transfaunation is performed. 3. Rumen transfaunation for digestive disorders 3.1. Simple indigestion A clinical sign of simple indigestion in dairy cattle is anorexia (reduction in appetite) [18] with ruminal hypomotility to atony (stasis) [6]. Sudden changes in dietary ingredients may initiate anorexia in ruminants [4] that are reflected in changes in rumen pH [6]. For example, changes in dietary ingredients that contribute to rapid lactic acid production impact rumen pH and populations of rumen microorganisms, in particular a decrease in rumen protozoa with increase acidity. Steen [19] provided diagnostic criteria for indigestion that included (1) ketotest (test for ketones) of 0 or 1 and (2) one of the following rumen fluid parameters including (a) a methylene blue reduction time > 3 min, (b) few large or small protozoa, or (c) reduced protozoal activity. Even though transfaunation of rumen fluid from a healthy door animal to an animal with simple indigestion is a common recommended practice for dairy cattle and other ruminants, there is little information on the practice in the scientific literature. Jasmin et al. [20] reported the beneficial effects of rumen transfaunation for sheep used in biomedical research that developed simple indigestion. In their biomedical research sheep were fed pelleted diets, which contributed to the development of subclinical rumen acidosis. Exacerbating the effects of the small particle size as well as grain content associated with the pelleted diet, there were also stresses E.J. DePeters, L.W. George / Immunology Letters 162 (2014) 69–76 associated with shipping and indoor housing, increased handling, fasting for experimental procedures, and perioperative opioids for pain management – all factors that were implicated by the authors with contributing to rumen atony and the manifestation of subclinical acidosis. To mimic the effect of indigestion, sheep were fed a completely pelleted diet. Postoperatively two sheep on the pelleted diet displayed rumen atony, feed intake was reduced by greater than 50%, and protozoal motility was reduced by 25–50%. Rumen transfaunation of one sheep once with 750 mL of fresh rumen contents returned the recipient animal to normal health although the other recipient animal required a second transfaunation with 750 mL of rumen fluid 24 h after the first treatment before returning to normal. Unfortunately, these authors did not measure rumen pH and volatile fatty acids post-transfaunation of the two sheep that recovered from simple indigestion. Regardless, rumen transfaunation corrected simple indigestion in sheep. It is common knowledge in the popular literature that rumen transfaunation for simple indigestion is effective treatment [21]. Simple indigestion can be an issue during early lactation of the transition period when dietary changes require dairy cows to adjust to diets high in nonstructural carbohydrates. During the transition period around calving cows experience not only diet changes, but also physiological changes associated with parturition (process of giving birth). Cows are also moving from different pens so social structure is changing and this can be a stressor. Tankersley et al. [22] used rumen transfaunation in the transition period of dairy cows in a field study with 210 Holstein dairy cows. Four ruminally fistulated, lactating dairy cows receiving a total mixed-ration of concentrate and forage were used as donor animals. These four ruminally fistulated cows were managed similar to all other lactating cows and were provided no special considerations. Rumen fluid transfaunation was one of four treatments applied where 11.4 L of rumen fluid were transferred by oral stomach-tube into the rumen of fresh cows (donor cows) approximately 24-h post calving. Treatments were (1) control – no oral supplement, (2) warm water via stomach tube, (3) commercial product via stomach tube, and (4) rumen fluid via stomach tube. The hypothesis was that rumen fluid transfaunation would improve health of cows after calving. Compared with control, all oral treatments did not affect serum analytes, milk yield, and animal health in a well-managed herd. However, a similar study should be done in a herd experiencing health and performance issues to evaluate the impact of rumen transfaunation. 3.2. Left sided abomasal displacement Rumen transfaunation was used as an adjunctive treatment following surgery. Rager et al. [23] transfaunated cows following surgical repair of left-displaced abomasum (LDA), a condition that is a 180-degree torsion of the abomasum occurring without volvulus (no restriction of digesta passage out of the abomasum). Rumen fluid (10 L) obtained from two non-lactating, ruminally fistulated donor cows fed a forage diet (e.g. diet composed of predominately hay) was transferred by stomach tube (oral) within 20 min of rumen fluid collection. Control cows received 10 L of lukewarm tap water also by stomach tube. Treatments occurred immediately following surgery and again on day 1 after surgery. Beginning on day 2 following surgery and for the next three days, rumen transfaunated cows had higher dry matter intake and milk yield compared with control cows. Rumen fluid pH and total concentration of volatile fatty acids did not differ for day post-surgery or between rumen transfaunated and control treatments. Serum concentrations of hydroxybutyrate on days 3 and 5 post-surgery were significantly lower in transfaunated cows than control cows. The authors concluded “administration of rumen fluid to cows convalescing after surgical correction of LDA had beneficial effects”. 71 4. Recommended method for rumen transfaunation Recommendations in the literature vary for rumen transfaunation, but a brief summary follows. Rumen transfaunate can be obtained during abattoir slaughter or at post mortem. However, using a non-screened donor may introduce infectious agents including Mycobacterium avium var paratb, Salmonella, Cryptospordia, or Escherichia coli (0157:H7). Other methods of transfaunate collection include stomach tube (oral or nasal passage of the tube), cud transfaunation, or direct removal from a donor with a surgically implanted rumen fistula [3–5,7]. Intubation and cud transfers provide limited volume, but may be preferable to having a known disease free source over a random abattoir collected sample. The efficacy of using a cud to transfaunate an adult animal is unknown, and may not provide sufficient microorganisms and substrates for full therapeutic benefit [4]. Mould et al. [24] cautioned that the microbial quality of rumen fluid collected prior to slaughter could be impacted by the fact that animals are often restricted in water and feed intake. The clinical implications of fasting related changes in rumen fluid are unknown. Radostits et al. [18] suggested that 20–30 L of water should be pumped into the rumen via stomach tube first followed by allowing the rumen fluid to flow out of the animal by siphon through the tube. Typically rumen fluid is transferred to the sick animal via a stomach tube, but an oral drench can be used [18]. Collection of large volumes of rumen fluid is most easily accomplished using a rumen fistulated animal. Removal of rumen fluid is less stressful when the donor is fistulated than by stomach tubing a non-fistulated donor. A rumen fistulated donor can be restrained with a halter while use of a stomach tube usually requires the nonfistulated donor to be restrained in a chute. Typically fistulated animals are housed and managed within the herd so there is limited risk of introducing unknown diseases and the rumen fluid reflects the diets fed. Volume of rumen fluid transferred ranges from 1 L for calves and 16 L for adult cattle [2–5,16]. For adult cattle 8–16 L was considered “ideal” [2,4,16]. Volume of transfaunate in clinical trials with adult dairy cattle ranged between 10 and 11.4 L per oral dose [22,23]. Smaller volumes ranging between 1 and 4 L could be used for goats and sheep (authors’ recommendation) while approximately 1 L was recommended for sheep [6]. Rumen fluid should be transferred as soon as possible postcollection [5]. Some authors suggested that the rumen fluid should be transferred to the recipient animal within 30 min of collection [7]. But others suggested that rumen fluid can be stored for up to 9 h at room temperature and for 24 h at refrigeration temperature [3,5]. Radostits et al. [18] reported that rumen fluid can be maintained at room temperature for several days. Lyophilized rumen fluid was used successfully in culture media [25], but freeze drying would not likely be a practical on-farm approach. Mould et al. [24] reported that previous work demonstrated that failure to maintain an anaerobic environment resulted in a decrease in ciliate protozoa numbers and a decrease in cellulolytic and amylolytic activity of the rumen fluid. However, if a container is sealed to reduce exposure to air, pressure could increase CO2 in the fluid and decrease pH. Hervás et al. [26] evaluated storage conditions for rumen fluid collected from sheep using in vitro methods. Storing rumen fluid under CO2 at 0 ◦ C for 3–6 h did not affect fermentation characteristics. In contrast, storing at 0 ◦ C for 24 h decreased fermentation characteristics. However, it should be noted that these researchers collected rumen fluid from sheep after an overnight fast so the restriction of feed and water could have impacted the quality of the rumen fluid used. In general, the sooner the collected rumen fluid is transferred from the donor to recipient the better. Best practice would be to transfer the rumen fluid as soon as possible. 72 E.J. DePeters, L.W. George / Immunology Letters 162 (2014) 69–76 The pH of the rumen fluid should be 5.5 or greater [7] and preferably 6.0 or greater. Rumen protozoa are decreased at low pH [15]. Typically rumen fluid pH decreased 2–4 h after feeding with high starch diets to lactating cows [27] so avoid collecting rumen fluid within about 4 h of feeding when the donor is receiving forage and concentrate ingredients. With non-lactating cows fed a high forage diet, rumen fluid collected from a fistulated cow can be done prior to feeding or about 2–3 h after feeding since pH does not change dramatically [28]. When using lactating cows with rumen fistulas, rumen fluid collection prior to feeding will likely provide rumen fluid above 6.0 pH. Lactating dairy cows eat throughout the day, but typically the largest consumption occurs after milking when cows are fed so collecting rumen samples after 4 h following a large intake of feed will minimize the chance collecting rumen fluid below 6.0 pH. Rumen fluid can be easily checked for pH using either a portable pH meter or pH paper. On average the pH of rumen fluid will be higher if the animal is consuming an all forage diet compared with a diet of forage and concentrate. Forage type and particle size will also have an impact when comparing hay versus silage fed to the donor animal. Mould et al. [24] discussed timing of rumen fluid collection relative to use of the fluid for in vitro inoculum, and many of these considerations apply to rumen transfaunation. Filter or strain large particulate matter from the rumen fluid using cheese cloth [3] or a large screen (authors’ recommendation) to remove large particles that can plug the stomach tube. The filtered material should contain small particles and their associated attached bacteria, protozoa, and fungi. Color of the rumen fluid will vary with diet. Garry [16] described the rumen fluid as olive to brownish green when cattle are eating hay and yellowish brown when cattle are eating silage or grain. Avoid using rumen fluid that is frothy or foamy (authors’ recommendation). Microscopic examination of protozoa can be performed if desired. Protozoa are large enough to see with a transition microscope and the motility of the ciliated protozoa is readily apparent. Methylene blue reduction time can be measured [4] by adding 0.5 mL of 0.03% methylene blue to 10 mL of ruminal fluid in a test tube followed by mixing. The rumen environment is highly reduced (high [H+ ] concentration). The blue color of the methylene blue should disappear within 2–6 min [4] and if the time require is greater than 10–15 min, it might be best to discard this rumen fluid (Fig. 3). A robust microbial population will reduce methylene blue to a colorless form. For cattle experiencing simple indigestion, in addition to rumen transfaunation, offer grass hay or straw because these are preferred over alfalfa hay or concentrate [4]. The experiences of the authors of this review are that cattle prefer oat hay over alfalfa hay when experiencing simple indigestion. Rumen fluid can be easily collected from a ruminally fistulated donor animal by creating a siphon (Fig. 2). The collected rumen fluid is best manually pumped into the recipient using a large polyethylene or metal stomach tube and a marine water pump. There are commercially available tubes and pumps available with a common pump system called “The Magrath Cattle Pump System”. 5. Rumen transfaunation in a research setting Williams and Withers [29] studied the re-introduction of ciliated protozoa following rumen transfaunation of defaunated sheep. Within 11 days of rumen transfaunation, ciliate protozoa recolonized the rumens of all sheep. Even though protozoa are reported to decrease bacterial populations due to competition for nutrients and predation, bacterial as well as fungal populations were not affected by the recolonization of the rumens by protozoa. Imai et al. [30] transferred rumen fluid from Japanese Sika deer that Fig. 2. Collection of rumen fluid from a rumen-fistulated donor and subsequent transfer of strained rumen-fluid to a recipient animal. E.J. DePeters, L.W. George / Immunology Letters 162 (2014) 69–76 73 It required many days, 61 days for one recipient, for the bacterial community composition to return to preexchange. 6. Application of rumen transfaunation for plant toxicants Fig. 3. Methylene blue test. contained nine species of ciliate protozoa into unfaunated shorthorn calves. All species of ciliates were present in calves by 14-days post transfaunation. Weimer et al. [31] ‘exchanged’ greater than 95% of the ruminal contents of the recipient animal with host contents to study bacterial communities. Within about 24 h the pH and total VFA concentrations in the recipient returned to preexchanged values. An interesting application of rumen transfaunation is the story related to the degradation of mimosine (␣-amino--(N-[3hydroxy-4-pyridone]) propionic acid). Mimosine is a toxic amino acid found in plants of the genera Leucaena and Mimosa [32,33]. Mimosine inhibits protein synthesis and when consumed longterm by animals, it resulted in reduced growth and loss of hair with presumed antimitotic activity [33]. Mimosine was degraded in the rumen to 3-hydroxy-4-pyridone [33], and in mice and rats this metabolite of mimosine was a goitrogen [34]. Mimosine is present in leguminous plants that grow in Australia and Hawaii [32]. Cattle and goats grazing in Australia had the rumen microflora to degrade mimosine but not its toxic metabolite, 3,4-dihydroxy pyridine. In contrast, leucaena was not toxic to ruminants in Hawaii and Indonesia because the rumen microflora could degrade both mimosine and 3,4-dihydroxy pyridine [35]. Jones and Megarrity [32] isolated and cultured microorganisms capable of degrading 3,4-dihydroxy pyridine from the rumen of a goat in Hawaii. A bacterial culture was developed and then taken to Australia where it was infused into one goat via a rumen fistula and one steer via a stomach tube. The diet of both animals contained leucaena and both animals prior to the infusion had 3,4-dihydroxy pyridine in their urine. Following the infusion of the bacterial culture, both animals had essentially no 3,4-dihydroxy pyridine in their urine and in vitro studies with rumen fluid obtained from the goat demonstrated that 3,4-dihydroxy pyridine was degraded. In a subsequent study by Quirk et al. [35] cattle were either dosed with a bacterial culture capable of degrading 3,4-dihydroxy pyridine or left untreated while grazing pastures containing leucaena. Dosed steers had greater live weight gain by week 19 of the study. However, after 19 weeks, the introduced bacteria were present in the untreated cattle. After week 19 the untreated cattle had low levels of 3,4-dihydroxy pyridine in their urine and their rumen fluid degraded 3,4-dihydroxy pyridine in vitro. Even though the pastures were designed to reduce the chance of transferring the introduced bacteria to the untreated group, transfaunation occurred. These scientists attributed the transfaunation of the untreated animals to the possibility of airborne spores from the feces of treated animals. More recently rumen fluid from goats with rumen bacteria capable of degrading sodium monofluoroacetate, a toxic compound in Amorimia spp., was used to transfaunate susceptible animals as a method to reduce animal poisoning [36]. Sodium monofluoroacetate was a possible link to sudden death in Brazilian cattle that consumed toxic plants. An interesting aspect of monofluoroacetate is that it inhibited methanogenesis in an anaerobic sample of rumen fluid [37]. The diversity of microbes and their ability to detoxify plant toxins will continue to be of interest in the future. Tannins are a group of polyphenolic compounds found in plants. Tannins are antimicrobial to some species of microorganisms and were shown to reduce methane production in sheep and goats [38]. Tannin consumption can also have harmful effects on animal health [39]. Tannins bind proteins and efforts are underway to use this binding property to reduce protein degradation in the rumen to enhance nitrogen utilization by ruminants [40] and to reduce bloat of cattle grazing alfalfa [38]. However, if tannins are to be effective it might require that they be degraded in the rumen. Rumen bacteria were isolated from several wild East African ruminants that were tolerant in culture to tannin [41]. Rumen cultures of selected domestic and wild ruminants in Ethiopia partially degraded tannins in in vitro cultures [42]. These researchers also isolated fecal microorganisms 74 E.J. DePeters, L.W. George / Immunology Letters 162 (2014) 69–76 from dikdik that completely degraded hydrolyzable tannin. Fecal microorganisms from a number of wild ruminants had the ability to completely degrade tannins [43]. Transfaunation of ruminants grazing plants containing tannins with tannin degrading microorganisms might be a potential approach to mitigate the impact of tannins on feed intake. Even though there are situations of establishment of bacteria in the rumen to deal with specific plant compounds, Weimer et al. [31] reported that attempts to introduce specific bacterial strains into the rumen have been, for the most part, unsuccessful due to host specificity. 7. Rumen fistula techniques There are numerous publications on the surgical procedures involved in rumen fistulation and we cite only a few [5,44–49]. Detailed instructions with photographs are published as an internet document. http://www.bardiamond.com/Library.php. http://www.bardiamond.com/Surgery Animal Care.html. http://www.bardiamond.com/uploads/Rumen Fistula SurgeryCattle Bar DiamondTM.pdf. The procedure is relatively simple and authors of this paper have done numerous rumen fistulations in cattle, sheep, and goats. Basically a circular incision is made into the skin and external abdominal oblique muscle. The circular part of the skin and muscle are removed. This is followed by a grid incision into the internal oblique and transversus abdominis muscle in cattle. The peritoneum is incised sharply. The rumen wall is exteriorized and is sutured to the muscular walls and peritoneum using overlapping mattress sutures. The rumen wall is then sutured to the skin edge, and the wound is covered with sterile gauze for 72 h. After that time the circular part of the rumen wall is removed and the cannula is inserted. However, it is also possible to immediately remove the circular part of the rumen wall once the rumen wall is sutured to the skin edge and then insert the cannula. One major concern during surgery is keeping the fistula opening size so the cannula fits snugly. If the fistula opening is too large, the cannula can fall out of the rumen or in some cases it can fall into the rumen. If it falls into the rumen, the cannula can often be found in the reticulum. If the cannula falls out a less flexible inner washer can be used. Tankersley et al. [22] used four ruminally fistulated, lactating dairy cows on a 1500 cow (milk herd size) dairy with no problems. The rumen fisulated cows were managed similar to any other cow in the herd with no special considerations. The current cannulas are made of soft material and designed to come out if a cow should catch the cannula on an obstruction, for example a cable. The cannula is easily re-inserted into the fistula. At the U.C. Davis dairy facility, the rumen cannulas are routinely replaced about every 18 months in the fistulated cows since they begin to break down with age and exposure to sunlight. The surgeries [22] were performed by the herd veterinarian in less than 60 min per animal. Point of interest: Loosli [50] stated that “rumen fistulas were first mentioned by Fluorens in 1833”. However, the first animal to be reported in the literature with a fistula into the stomach compartment might have been a human by the name of Alexis St. Martin [45]. The story various depending on the source, but one version is that in June of 1822, an army surgeon by the name of William Beaumont was called to treat a French Canadian voyager, Alexis St. Martin, who was shot accidently with a shotgun. St. Martin survived but the skin and stomach wall had healed creating a permanent opening, fistula, into his stomach compartment. Over the next 8 years, Beaumont conducted studies on gastric physiology. Many of the aspects of gastric physiology that we know today come from the work of Beaumont. For example, the stomach was observed to secrete acid in response to the presence of food in the stomach and not produced constantly as was the thought at that time. Beaumont also noted that mucous was secreted and secreted separately from acid. Beaumont conducted over 100 different studies on St. Martin, who lived into his 80s (depending on source maybe 86) and he fathered numerous children (depending on source maybe 17). The stomach fistula did not impact St. Martin’s life span as far as we know, and the same happens for rumen fistulated dairy cattle that typically live beyond 10 years of age at U.C. Davis. 8. Intrinsic aspects of transfaunate Rumen fluid contains many chemical constituents that likely contribute to the beneficial effects of transfaunation. Such factors could include volatile fatty acids, bicarbonate buffers, proteins, and intact microbes although there are many unidentified constituents in rumen fluid. The effects of mechanical stimulation by bulk activity of the transfaunate may also be beneficial. Moderate mechanical distension in the area of the reticulum and reticulo-rumen fold can excite tension receptors [51] located in the walls of the rumen and reticulum [52]. Leek [51] stated that there could also be “unknown chemical stimulation arising from fermentation” that caused muscular contractions. Transfaunation with 8–16 L of rumen fluid in cattle could induce mechanical stimulation of tension receptors to stimulate rumination, salvation, and normal rumen motility. The effects of osmolal changes in recipients following transfaunation are unknown. Bryant and Doetsch [53] demonstrated that Bacteroides succinogenogenes, cellulolytic bacteria, required factors in rumen fluid for growth. These factors were branched-chain and straight-chain saturated acids. Subsequent research demonstrated that ammonia was essential for growth even in the presence of amino acids [54]. These researchers also found that biotin and selected minerals were necessary for growth of B. succinogenogenes. Streptococcus bovis ferments starch, is acid tolerant, and can grow rapidly. Under “normal conditions” S. bovis produced acetate, formate, and ethanol [55]. In response to excess readily rumen digestible carbohydrates, S. bovis yielded lactate, which contributes to subclinical acidosis associated with diet changes and indigestion. S. bovis required biotin, thiamine was stimulatory, and some strains were reported to require the B vitamins, pantothenic acid and nicotinic acid [9]. Ammonia could serve as the nitrogen source. There is a cross feeding of nutrients among the microorganisms that live in the rumen [56,57]. Miura et al. [57] described it as nutritional interdependence where, for example, the branch-chain fatty acid produced by one bacterial group was used by branch-chain fatty acid dependent groups for growth. There was reported to be considerable cross feeding of cellulose degradation products in the rumen [58]. Likewise, the B vitamins produced by some organisms are required for growth by other organisms. These are just a few examples of how complex the rumen environment is and how interlinked the microorganisms can be. The nutrients in rumen fluid transfaunate will likely benefit the growth of bacteria, protozoa, methanogens, and fungi to restore normal microflora populations. Protozoa in the rumen transfaunate likely play an important role in re-establishing the rumen microbial population. Protozoa concentration increased as the proportion of starch or sugar in the diet increased [59]. Protozoa were reported [59] to stabilize the rumen pH in response to the consumption of readily available starch and to decrease the redox potential. Consequently these actions create a rumen environment conducive to cellulolytic bacteria growth. Protozoa also degrade protein and contribute peptides that stimulate the growth of other microorganisms. E.J. DePeters, L.W. George / Immunology Letters 162 (2014) 69–76 The intrinsic factors associated with rumen fluid are not well described or understood, but there are obvious benefits to the animal. Technology is now available to study ruminal fluid metabolome [60] and improve our understanding. 9. Fecal microbiota transplantation Transfaunating the rumen of sick animals using the microorganisms obtained from a healthy donor is common practice. Microbial transfer is also used in human medicine. The transfer of beneficial fecal microorganism (fecal microbiota transplantation; FMT) was reported to be practiced for more than 40–50 years [61,62]. The concepts for ruminants and humans are similar – support a healthy microbiology population in the digestive system and there is a healthy host. Humans can be thought of as superorganisms because there is a vibrant microbial population in symbiotic relation with the host. Microbial floral was re-established in patients suffering from Pseudomembraneous enterocolitis [63]. Borody and Khortus [62] discussed the benefit of FMT with respect to Clostridium difficile infection following the use of antibiotics that disrupted the microbial community. Treatment of C. difficile involved antibiotics, but relapses occurred. Cure rates were high using FMT [64,65]. Following FMT the bacterial population of the recipient reflected the bacterial population of the donor [66,67]. Bakken [68] used what was called fecal bacteriotherapy to successfully cure C. difficile (90% success rate). The fecal liquid suspension from a donor was transferred into a recipient “from either end of the GI tract”. 10. Summary Rumen transfaunation is a routine, widely accepted, successful procedure to treat simple indigestion in ruminants. The procedure also has clinical application for post-operative treatment of cattle with left sided abomasal displacements. Rumen fluid from a healthy donor provides the recipient with diverse microorganisms that can repopulate the rumen. Transplanted rumen fluid also provides nutrients and energy to the rumen microbial population. Although widely accepted as a treatment for simple indigestion, there is a paucity of information in the literature to describe the mechanisms involved in the beneficial effects of rumen transfaunation. References [1] Brag S, Hansen HJ. Treatment of ruminal indigestion according to popular belief in Sweden. Rev Sci Tech Off Int Epiz 1994;13:529–35. [2] Constable PD. The ruminant forestomach. In: Howard JL, Smith RA, editors. Current veterinary therapy. Food animal practice. Philadelphia, PA, USA: W.B. Saunders Company; 1999. p. 502–7. [3] Garry FB. Rumen indigestion and putrefaction. In: Anderson DE, Rings DM, editors. Current veterinary therapy, food animal practice. Saunders, Elsevier: St. Louis, MO; 2009. p. 20–3 [chapter 7]. [4] Grunberg W, Constable PD. Function and dysfuntion of the ruminant forestomach. In: Anderson DE, Rings DM, editors. Current veterinary therapy, food animal practice. St. Louis, MO: Saunders, Elsevier; 2009. p. 12–9 [chapter 6]. [5] Laflin SL, Gnad DP. Rumen cannulation: procedure and use of a cannulated bovine. Vet Clin Food Anim Pract 2008;24:335–40. [6] Merck veterinary manual. 10th ed. Whitehouse Station, NJ, USA: Merck & Co., Inc. p. 199–200, 2010 2183–8. [7] Shanks M. Transfaunation – a practical technique for the bovine practitioner. Cattle Pract 2012;20:81–3. [8] Smith BP. Large animal internal medicine: diseases of the alimentary tract. In: Murray MJ, editor. Large animal internal medicine. 4th ed. USA: Mosby, St. Louis, MO; 2009. [9] Hungate RE. The rumen and its microbes. New York, NY, USA: Academic Press, Inc.; 1966. [10] Pounden WD, Hibbs JW. The influence of the ration and rumen inoculation on the establishment of certain microorganisms in the rumens of young calves. J Dairy Sci 1948;31:1041–50. [11] Pounden WD, Hibbs JW. The influence of pasture and rumen inoculation on the establishment of certain microorganisms in the rumen of young calves. J Dairy Sci 1949;32:1025–31. 75 [12] Conrad HR, Hibbs JW, Pounden WD, Sutton TS. The influence of rumen inoculations on the digestibility of dry matter, cellulose and protein in young dairy calves. J Dairy Sci 1950;33:378. [13] Pounden WD, Hibbs JW. Rumen inoculations in young calves. J Am Vet Med Assoc 1949;114:33–5. [14] Dehority BA. Specificity of rumen ciliate protozoa in cattle and sheep. J Protozool 1978;25:509–13. [15] Williams AG, Coleman GS. The rumen protozoa. Brock/Springer series in contemporary bioscience New York: Springer-Verlag New York Inc.; 1992. [16] Garry F. Diagnosing and treating indigestion caused by fermentative disorders. Vet Med 1990;85:660–70. [17] Ffoulkes D, Leng RA. Dynamics of protozoa in the rumen of cattle. Br J Nutr 1988;59:429–36. [18] Radostits OM, Gay CC, Blood DC, Hinchcliff KW. Veterinary medicine. A textbook of the diseases of cattle, sheep, pigs, goats and horses. 9th ed. London: W.B. Saunders; 2000. [19] Steen A. Field study of dairy cows with reduced appetite in early lactation: clinical examinations, blood and rumen fluid analyses. Acta Vet Scand 2001;42:219–28. [20] Jasmin BH, Boston RC, Modesto RB, Schaer TP. Perioperative ruminal pH changes in domestic sheep (Ovis aries) housed in a biomedical research setting. J Am Assoc Lab Anim Sci 2011;50:27–32. [21] Dumas C. Heal thyself – this dairy treats its own with help of wonder cow. Dairy Today 1999;(September issue):18. [22] Tankersley NS, DePeters EJ, Graham TW. Case study: effects of water, fresh cow YMCP plus, and rumen fluid transfaunate supplementation following calving on milk yield, reproductive efficiency, and incidence of common health disorders in Holstein cows. Prof Anim Scient 2007;23:513–20. [23] Rager KD, George LW, House JK, DePeters EJ. Evaluation of rumen transfaunation after surgical correction of left-sided displacement of the abomasum in cows. J Am Vet Med Assoc 2004;2004(225):915–20. [24] Mould FL, Kliem KE, Morgan R, Mauricio RM. In vitro microbial inoculum: a review of its function and properties. Anim Feed Sci Technol 2005;123–124:31–50. [25] Dehority BA, Tirabasso PA. Lyophilization of rumen fluid for use in culture media. Appl Environ Microbiol 1989;55:3237–9. [26] Hervás G, Frutos P, Giráldez FJ, Mora MJ, Fernández B, Mantecón AR. Effect of preservation on fermentative activity of rumen fluid inoculum for in vitro gas production techniques. Anim Feed Sci Technol 2005;123–124: 107–18. [27] Joy MT, DePeters EJ, Fadel JG, Zinn RA. Effects of corn processing on the site and extent of digestion in lactating cows. J Dairy Sci 1997;80:2087–97. [28] DePeters EJ, Fadel JG, Arosemena A. Digestion kinetics of neutral detergent fiber and chemical composition within some selected by-product feedstuffs. Anim Feed Sci Technol 1997;67:127–40. [29] Williams AG, Withers SE. Changes in the rumen microbial population and its activities during the refaunation period after the reintroduction of ciliate protozoa into the rumen of defaunated sheep. Can J Microbiol 1993;39: 61–9. [30] Imai S, Matsumoto M, Watanabe A, Sato H. Establishment of a spinated type of Diplodinium rangiferi by transfaunation of rumen ciliates of Japanese Sika Deer (Cervus nippon centralis) to the rumen of two Japanese Shorthorn calves (Bos taurus taurus). J Eukaryot Microbiol 2002;49:38–41. [31] Weimer PJ, Stevenson DM, Mantovani HC, Man SLC. Host specificity of the ruminal bacterial community in the dairy cow following near-total exchange of ruminal contents. J Dairy Sci 2010;93:5902–12. [32] Jones RJ, Megarrity RG. Successful transfer of DHP-degrading bacteria from Hawaiian goats to Australian ruminants to overcome the toxicity of leucaena. Aust Vet J 1986;63:259–62. [33] Soedarjo M, Hemscheidt TK, Borthakur D. Mimosine, a toxin in leguminous trees (Leucaena spp.), induces a mimosine-degrading enzyme activity in some rhizobium strains. Appl Environ Microbiol 1994;60:4268–72. [34] Hegarty MP, Lee CP, Christie GS, Court RD, Haydock KP. The goitrogen 3hydroxy-4(1H)-pyridone, a ruminal metabolite from Leucaena leucocephala: effects in mice and rats. Aust J Biol Sci 1979;1979(32):27–40. [35] Quirk MF, Bushnell JJ, Jones RJ, Megarrity RG, Butler KL. Live-weight gains on leucaena and native grass pastures after dosing cattle with rumen bacteria capable of degrading DHP, a ruminal metabolites from leucaena. J Agric Sci (Cambridge) 1988;111:165–70. [36] Duarte AL, Medeiros RMT, Riet-Correa F. Poisoning by Amorimia spp. in ruminants. Cienc Rural Santa Maria 2013;43:1294–301. [37] Emptage M, Tabinowski J, Odom JM. Effect of fluoroacetates on methanogenesis in samples from elected methanogenic environments. Environ Sci Technol 1997;31:732–4. [38] Patra AK, Saxena J. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J Sci Food Agric 2011;91:24–37. [39] Belenguer A, Hervás G, Yáñez-Ruiz DR, Toral PG, Ezquerro C, Frutos P. Preliminary study of the changes in rumen bacterial populations from cattle intoxicated with young oak (Quercus oyrenaica) leaves. Anim Prod Sci 2010;50:228–34. [40] Getachew G, DePeters EJ, Pittroff W, Putnam DH, Dandekar AM. Review: does protein in alfalfa need protection from rumen microbes? Prof Anim Scient 2006;22:364–73. [41] Odenyo AA, Bishop R, Asefa Jamnadass G, Odongo D, Osuji P. Characterization of tannin-tolerant bacterial isolates from East African Ruminants. Anaerobe 2001;7:5–15. 76 E.J. DePeters, L.W. George / Immunology Letters 162 (2014) 69–76 [42] Ephraim E, Odenyo A, Ashenafi M. Screening for tannin degradation by rumen and faecal samples of wild and domestic animals in Ethiopia. World J Microbiol Biotechnol 2005;21:803–9. [43] Ephraim E, Odenyo A, Ashenafi M. Isolation and characterization of tannindegrading bacteria from faecal samples of some wild ruminants in Ethiopia. Anim Feed Sci Technol 2005;118:243–53. [44] Dougherty RW. Fistulas and pouches in ruminants. In: Experimental surgery in farm animals. Iowa State University; 1981 [chapter 6]. [45] Dougherty RW. Permanent stomach and intestinal fistulas in ruminants: some modifications and simplifications. Cornell Vet 1955;45:331–57. [46] Komarek RJ. Rumen and abomasal cannulation of sheep with specifically designed cannulas and a cannula insertion instrument. J Anim Sci 1981;53:790–5. [47] Saeed A, Pir-Mohammadi R, Pour-Hasani F. A two-stage rumen cannulation technique in sheep. J Anim Vet Adv 2007;6:29–32. [48] Muzzi LAL, Muzzi RAL, Gabellini ELA. Rumen fistulation and cannulation technique in cattle and sheep. Cienc Agrotecnol 2009;33:2059–64. [49] Szakacs J, Chrastinova L, Vancisin J, Klega K. Cannulation of rumen in cattle. Vet Med 1990;35:449–55. [50] Loosli JK. History of the development of animal nutrition. In: Putnam PA, editor. Handbook of animal science. San Diego, CA: Academic Press, Inc.; 1991. p. 26–48. [51] Leek BF. Clinical diseases of the rumen: a physiologist’s view. Vet Rec 1983;1983(113):10–4. [52] Forbes JM, Barrio JP. Abdominal chemo- and mechanosensitivity in ruminants and its role in the control of food intake. Exp Physiol 1992;77:27–50. [53] Bryant MP, Doetsch RN. Factors necessary for the growth of Bacteroides succinogenes in the volatile acid fraction of rumen fluid. J Dairy Sci 1955;38:340–50. [54] Bryant MP, Robinson IM, Chu H. Observations on the nutrition of Bacteroides succinogenes – a ruminal cellulolytic bacterium. J Dairy Sci 1959;42: 1831–47. [55] Herrera P, Kwon YM, Ricke SC. Ecology and pathogenicity of gastrointestinal Streptococcus bovis. Anaerobe 2009;15:44–54. [56] Krause DO, Nagaraja TG, Wright ADG, Callaway TR. Board-invited review: rumen microbiology: leading the way in microbial ecology. J Anim Sci 2013;91:331–41. [57] Miura H, Horiguchi M, Ogimoto K, Matsumoto T. Nutrition interdependence among rumen bacteria during cellulose digestion in vitro. Appl Environ Microbiol 1983;45:726–9. [58] Russell JB, Hespell RB. Microbial rumen fermentation. J Dairy Sci 1981;64:1153–69. [59] Jouany JP, Ushida K. The role of protozoa in feed digestion – review. AsianAustralas J Anim Sci 1999;12:113–28. [60] Saleem F, Bouatra S, Guo AC, Psychogios N, Mandal R, Dunn SM, et al. The bovine ruminal fluid metabolome. Metabolomics 2013;9:360–78. [61] Borody TJ, Warren EF, Leis S. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol 2003;37:42–7. [62] Borody TJ, Khortus A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol 2012;9:88–96. [63] Bowden TA, Mansberger AR, Lykins LE. Pseudomembraneous enterocolitis: mechanism of restoring floral homeostatis. Am Surg 1981;47:178–83. [64] Borody TJ. Flora power – fecal bacteria cure chronic C. difficile diarrhea. Am J Gastroenterol 2000;95:3028–9. [65] Borody TJ, Campbell J. Fecal microbiota transplantation. Techniques, applications, and issues. Gastroenterol Clin North Am 2012;41:781–803. [66] Floch MH. Fecal bacteriotherapy, fecal transplant, and the microbiome. J Clin Gastroenterol 2010;44:529–30. [67] Grehan MJ, Borody TJ, Leis SM, Campbell J, Mitchell H, Wettstein A. Durable alteration of the coloni micrbiota by the administration of donor fecal flora. J Clin Gastroenterol 2010;44:551–61. [68] Bakken JS. Fecal bacteriotherapy for recurrent Clostridium difficile infection. Anaerobe 2009;15:285–9.