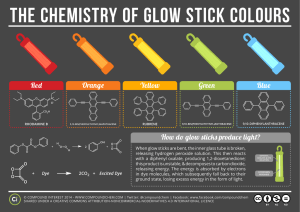
Unit 5: Glow sticks Experiment Essential Question: How does temperature affect the rate of a chemical reaction? Read the procedure below and then write your hypothesis: ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ Procedure: 1. Label 3 beakers with masking tape: Hot, Cold, Room Temp. 2. Measure and pour 200 ml of hot water, 200 ml of cold water, and 200 ml of tap water into the correctly labeled beaker. 3. Using a digital thermometer, take the temperature of the WATER in each labeled beaker. Record it in the data table below. 4. Break and “activate” 3 glow sticks of the same color. Shake to mix the chemicals for about 5 seconds to start the chemical reactions. 5. Simultaneously start the stopwatch AND place one glow stick in each beaker at the SAME TIME. 6. Record the difference in brightness as soon as you place the glow sticks in the water in the table below. Make sure to focus on the glow stick in the water. ( brighter, dimmer, same) 7. Observe the glow sticks and record the approximate TIME it takes to either become dimmer or stop glowing completely in the last column of your data table. PAY ATTENTION! 8. What is the Independent Variable?______________________________________ 9. What is the Dependent Variable ?_______________________________________ 10. What are 2 Controlled Variables?________________________________________ Glowstick color:__________________ Data Table: Quantitative Data Temperature (°C) Qualitative Data Observations (brightness) Quantitative Data Time to glow dimmer Hot Water Room Temp. Water Cold Water Challenge question: Based on your data, think of 1 -2 ways to make your glowsticks glow for longer. _____________________________________________________________________________________ _____________________________________________________________________________________ Conclusion: How does temperature affect the rate of a reaction? Write a scientific argument using the C-E-R Format below Claim: Answers the essential question ___________________________________________________________________________________ Evidence: Include quantitative and qualitative data from the table above. ____________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ Reasoning: Explain how your evidence proves your claim. Include how heat could affect the rate of a reaction. Include science concept(s) that would connect to your evidence and claim from the unit on chemical reactions. ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________