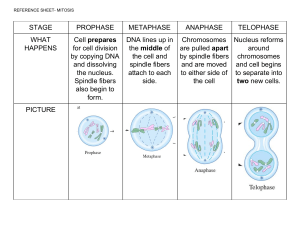
How many microtubules can attach to one chromosome at max during the mitosis and is it dependant on the organism ?" The number of microtubules attached to one kinetochore is variable. In the unicellular eukaryote, Saccharomyces cerevisiae only one microtubule binds each kinetochore, whereas in mammals there can be 15–35 microtubules s bound to each kinetochore. I have a doubt that "Is the aster's microtubules are the ones which depolymerize during the anaphase B or it is the kinetochore microtubules from the centrosome end which depolymerize during anaphase B? During Anaphase the sister chromatids separate and move towards the respective poles. This happens by shortening of the ‘kinetochore microtubules’. The shortening can happen by shortening at the kinetochore end or at the polar end. Experiments from different groups have shown that both possibilities exist : in different organisms, different mechanisms are found. The Mitotic Spindle during Metaphase An aster is a radial array of short microtubules Centrioles are involved in organizing the spindle microtubules Kinetochores are protein complexes associated with centromeres Spindle microtubules–during prometaphase, attach to kinetochore & begin to move chromosome Metaphase plate: an imaginary structure at the midway point between the spindle poles If two cells with G1 and M phases respectively and get fused, the cell in the G1 phase will directly go to the M phase directly without chromosome duplication? And what will happen if this fused cell divides? This is an artificial system, and cell with 2 nuclei will not survive. It is only an experiment to show that signals are present for these different phases. Can both anaphase A as well as Anaphase B happen together in the cell division, in some organisms? Anaphase A and Anaphase B are 2 different phases of Anaphase. In other words, Anaphase has been split up into Anaphase A and Anaphase B. Both will happen, but Anaphase A will occur first, followed by Anaphase B. Most books will simply refer it to as Anaphase which is also fine. You had mentioned that there can be 15-30 microtubules bound to each chromosome in mammals. Does each sister chromatid have an equal number of microtubules from the respective poles, or can they be unequal as well? I would assume so, because otherwise it might be difficult for them to be drawn equally. But I am not sure if any researcher has actually checked this point out. You had mentioned that cell organelles are fragmented during prophase, and they reassemble after cytokinesis in the daughter cells. So the daughter cells will also have an equal number of cell organelles as the parent cell. To achieve this, do cell organelles also undergo duplication during the synthesis phase? Yes, cell organelles are fragmented during prophase, and they reassemble after cytokinesis in the daughter cells. The exact numbers may not be identical. But what happens is that the fragmented organelles come together to grow in size, and all almost equivalent number. This happens mostly in the G1 phase. This is still an active field of research, so hope the answer is correct as of today. Does a chromosome always exist with two sister chromatids? Or is it so only after replication in the prophase and metaphase? Because in school I've learnt that a chromosome always has two sister chromatids attached at the centromere. That was the very basis of talking about centromere position and classifying chromosomes on that basis. No, a chromosome does not always exist with two sister chromatids. That happens only after the Replication of the DNA in the S Phase, when the duplicated chromosome is now referred to as the sister chromatids. Thus, The sister chromatids are seen Only after replication in the S phase. Then the replicated chromosome (original + copy)are attached to each other as sister chromatids Why it isessential for chromosomes to come on metaphase plate to split .? The Metaphase plate is an imaginary region that lies in the centre of the cell as it is dividing. Possibly the alignment on that plate allows it to move equally in either direction rather than unequally. Why spindle fibers don't connect directly to the centromeres? The centromeres are regions of the DNA around which the cohesins also bind. Possibly the spindle fibres don’t have access to the centromeres. So instead, what happens is that a ‘kinetochore complex of proteins forms on either side of the two sister chromatids. This is also at the centromeric region. The spindle fibres can bind easily to the kinetochores that are located towards the outside of the centromeric regions. Are all Protein Spindle Tubule fibres correctly bound to kinetochore ? If there are checkpoints and regulatory complexes in the cell cycle, then how chromosomal defects happen? What really these chromosomal defects bring changes in cell cycle? Chromosomal defects can happen either due to DNA damaging agents, chemicals. When these damages or problems are detected by the cell, the cyclin-dependant kinases are not able to function properly and thus do not allow the cells to move into the next phase What happens to the organelles during cell division. You said about the nucleus membrane and mitochondria, which I understood. NCERT says "Cells at the end of prophase, when viewed under the microscope, do not show golgi complexes, endoplasmic reticulum, nucleolus and the nuclear envelope." Does some chemical degrade them? If yes, why don't organelles get degraded during interphase? In mammalian cells organelles cannot be viewed under the microscope during the prophase not because they disappear, but because they fragment into smaller fragments. In other words they become dispersed/fragmented and during cytokinesis segregate into the two daughter cells, where they subsequently reassemble their normal interphase configuration What is the role of motor proteins during mitosis ? Is it only for spindle formation orfor other functions as well ? The motor proteins include the kinesins and dyneins Motor proteins seem to have a significant role not only in the spindle formation but in different stages ofmitosis.. The role of kinesins is a very active area of research. I give below some of the roles that kinesins participate in from a review on the subject: Kinesin-4Connects chromosomes to spindle, Kinesin-5 Establishes spindle bipolarity Kinesin-7 Links kinetochores to microtubules Kinesin-8 Subdues chromosome oscillation Kinesin-10 Connects chromosomes to spindle Kinesin-12 Maintains spindle bipolarity Kinesin-13 Depolymerises microtubules Kinesin-14 Restricts spindle length21 Movement of Chromosomes during Anaphase Nonkinetochore tubules from opposite poles overlap and push against each other elongating the cell In telophase: daughter nuclei form at opposite end Cytokinesis begins during anaphase or telophase, and spindle eventually disssambles "Signals relate not to single cell, but to the function of entire organism" what does this mean? Unlike unicellular eukaryotes like yeasts, most eukaryotic cells play distinct roles in the organism, and thus they do not have the freedom of unicellular cells to grow uncontrollably if the nutrient conditions are adequate. In Campbell at pg231 it is mentioned that "G1 is the most variable in length in different types of cell" what does this statement mean? It means that the time spent in G1 can be different for different cell types. In some cases it can be about 10 hours, but in other’s it might be less or more. In NCERT Book there are no prometaphase state... What is the actual significance of thisstate? Yes, many books do not refer to the protometaphase. Since the protometaphase can be distinguished under the microscope it has been designated as a separate phase. In this phase the sister chromatids are not yet aligned on the metaphase plate although they have begun to get linked to the microtubules. What is difference between sister chroamtid cohesion, centromere and kinetochore and what is actual positioning of these structure? Centromeric region of the DNA in the sister chromatids is where the cohesin protein forms a ring to keep the 2 chromatids together. The kinetochore is a protein complex that also binds to the centromeric region towards the outside of each of the sister chromatids and links each of the chromatids through microtubules to each of thespindle poles. Are all Protein Spindle Tubule fibres correctly bound to kinetochore ? How can an external signal cause the initiation of cell division in the eukaryotic cells except for the example, when we go to higher altitudes the rate of formation of RBCs increases, adding to it how do RBCs receive the signal? they don't even have a Nucleus. We will talk about signals in this class Where do the nucleolus, mitochondria and other organelles disappear while cell division, why is the need to do so since DNA has already been replicated and it has the sequence to form organelles itself except mitochondria? They don’t disappear, they fragment in prophase, and then grow in size again after cytokinesis Signals for Mitotic cell division ? Both external /Internal signals What are the Internal signals ? Signal: At G1 to S phase At G2 to M phase In M (SAC) Replication of DNA : S phase Segregation : M phase ( Mitosis) Cytokinesis : External Signals for a cell : Come from the surrounding tissues All cells do not constantly divide ( even when environmental conditions adequate) Most eukaryote cells : part of a multicellular organism & specialized Signals relate not to a single cell, but to the function of entire organisms Examples of differential behaviour of cells Human skin cells divide frequently throughout life Liver cells: Keep capability in reserve. Divide only when it is required. Injury Brain cells, Muscle cells : Do not divide Thus : Frequency varies with the type of cell Why, what determines the control ? Regulation occurs at the molecular level Cancer cells manage to escape the usual controls of the cell cycle Cancer Cells showed uncontrolled growth: Loss of control signals How can we demonstrate the presence of signals ? Are there really any internal signals that should tell the cell to divide ? How can we demonstrate this ? An experiment done to ask the question : is there a cytoplasmic signal that drives the cell signal ? Experiment : Take G1 phase cells : no DNA synthesis occurs here Fuse these G1 cells with S phase cells : make a single cell with 2 nuclei What happens ? How do you interpret the results of these experiments ? Nature, 1970 A second Experiment: Take G1 phase cells : no DNA synthesis occurs here Fuse these G1 cells with M phase cells : make a single cell with 2 nuclei What results can emerge ? What would be the interpretations ? ? The Cell Cycle has “Stop” and “Go” Signals - Called “Checkpoints” The 3 Important checkpoints are : - G1/S - G2/M - In M : SAC checkpoint (metaphase to anaphase) What happens at these checkpoints ? G1/S DNA Intact Favourable environmental conditions Provides time to repair damaged DNA G2/M DNA Intact DNA synthesis complete Cell size ? Prevents cells from dividing with damaged DNA Spindle Assembly Checkpoint : metaphase to anaphase) microtubule defects erroneous kinetochore attachment enter mitosis with damaged DNA Inactivation of the SAC can lead to chromosome mis-segregation and aneuploidy G1/S Checkpoint: For many cells, G1 checkpoint seems to be the most important If cells receive a ‘Go-ahead’ signal at the G1 checkpoint it will usually complete the S,G2 and M phases and divide If cells do not receive the Go-ahead signal, it will exit the cell cycle it will switch into a non-dividing state called the Go phase. Cell Cycle Controls Internal Signals Cyclins (proteins) Cyclin-dependant kinases (CDK) Proteins whose Conc Vary within Cell cycle CDKs activities Fluctuate because it is Controlled by cyclins Example: MPF: Cyclin-CDK that triggers cell’s Passage past G2 into M External Signals Growth Factors Density dependant inhibition Anchorage dependance Cyclins What is a cyclin ? A protein that ‘cycles’ during the cell cycle There are many different Cyclins : the protein levels go up and down with the cell cycle M Cyclins ( or B cyclins ) are maximum in M phase G1/S Cyclins are maximum in G1/S Boundary Different Cyclins reach Optimum Levels at different time How are the protein levels regulated ? G1/S = Cyclin E2 S = Cyclin A M = cyclin B - At the transcriptional level - By protein degradation Kinases What is a kinase ? A kinase is an enzyme that phosphorylates a protein target There are many different kinases in the cell Phosphorylation of a protein can change its function Cyclin Dependant Kinases (CDK) A special type of kinase whose activity depends on the binding to a specific cyclin It is a kinase that gets active when it binds to a specific cyclin Cdk protein levels Cyclin levels : up /down CDK levels : constant CDK activity : up/down Let us see how the G1/ S cyclin acts as a checkpoint Signal G1/S = Cyclin E2 The G1/S cyclin binds to its kinase, and the kinase becomes active The active ‘CDK Kinase’ then phosphorylates its targets, leading to signals How does DNA damage affect G1/S cyclin, and act as a checkpoint regulator ? MPF : Mitosis Promoting Factor is a Cyclin-dependant Kinase M (or B) Cyclin levels vary with the cell cycle. Most abundant at Mitosis MPF-dependant Kinase (MPF Cdk) protein levels remain constant MPF-dependant kinase (MPF CdK) activity changes during the cell cycle Molecular Mechanisms that help regulate the Cell cycle: G2/M G2/M : Checkpoint Cyclin B or Cyclin M Permitting entry into Mitosis CDK Levels remain constant , but gets activated by the Cyclin M ( or Cyclin B) M-cyclin bound to CDK activates this CDK. Activated CDK phosphorylates proteins, leads to entry into mitosis What about the Spindle Activation Checkpoint ? It allows cells to shift from Metaphase to Anaphase Does not Involve a CDK Ensures All the microtubules are bound to the kinetochores A “straggler” chromosome will lead to mitosis pausing Provides time for chromosomes to align before proceeding further Spindle checkpoint ensures that all is ‘OK’ before proceeding for Anaphase Metaphase Anaphase Are all Protein Spindle Tubule fibres correctly bound to kinetochore ? Anaphase Promoting Complex (Cyclosome) Ub= ubiquitin Cell Cycle Controls Internal Signals Cyclins (proteins) Cyclin-dependant kinases (CDK) External Signals Growth Factors Proteins Released by cells that Stimulate other cells to grow Density dependant inhibition After a certain density is reached cells stop growing Epidermal Growth Factor (EGF), Platelet derived growth factor (PDGF) Anchorage dependance Must be attached to a substratum in order to divide Platelet Derived Growth Factor (PDGF) allows growth of fibroblasts Anchorage-dependance Growth Density Dependant Inhibition Loss of Cell Cycle Controls in Cancer Cells Cancer Cells do not respond normally to the body’s control mechanism Cancer Cells may not need growth factors to grow and divide - They make their own growth factor - They may convey a growth factors signal without the presence of growth factor They may have an abnormal cell cycle control system Types of Cancer Cells A normal cell is converted to a cancerous cell by a process called transformation Cancer cells that are not eliminated by the immune system, form tumours, masses of abnormal cells within otherwise normal tissue Benign tumour: Abnormal cells remain at original site. can be removed by surgery Malignant tumours: Invade surrounding tissues and can metastatize Export cancer cells to other parts of the body where they form additional tumours How a Breast cancer can become metastatic Action of anticancer drugs in the cell cycle • restriction point: (G1 checkpoint) a point in the animal cell cycle at which the cell becomes “committed” to the cell cycle, which is determined by external factors and signals Cell-Cycle Checkpoints Cell-cycle checkpoints enable a cell to ensure that important processes, such as DNA replication, are complete [18]. Cell-cycle checkpoints prevent the transmission of genetic errors to daughter cells. There exist three major cell-cycle checkpoints; the G1/S checkpoint, the G2/M checkpoint, and the spindle assembly checkpoint (SAC). The SAC ensures that chromosome segregation occurs correctly and is activated at the metaphase to anaphase transition in mitosis, in response to microtubule defects [19] or an erroneous kinetochore attachment [20]. Cells also arrest at the SAC when they enter mitosis with damaged DNA [21]. Inactivation of the SAC can lead to chromosome missegregation and aneuploidy [22]. The G1/S and G2/M checkpoints are initiated in response to DNA damage to prevent the transmission of damaged or incomplete chromosomes to daughter cells. The DNA-damage checkpoints provide cells with time to repair damaged DNA. If the DNA damage is irreparable, cells may initiate senescence (growth arrest) or cell death. The G1/S checkpoint prevents cells from replicating damaged DNA, whereas the G2/M checkpoint prevents cells from dividing with damaged DNA [18]. The G1/S checkpoint does not function when p53 or p21 are either absent or not functional [23]. This checkpoint is often defective in cancer cells because many of them have mutations in the genes that encode either p53, pRb, or p21 [2,19]. This means that the only DNA-damage checkpoint available to these cancer cells is the G2/M checkpoint [18]. 3 The kinases ataxia telangiectasia mutated (ATM) and ATM and Rad3 related (ATR) are both involved in the initiation of the G2/M checkpoint, however ATR is the main effector kinase associated with G2/M arrest [24]. When single-stranded DNA (ssDNA) is present, it is bound by replication protein A (RPA) [25]. RPA recruits the ATR-interacting protein (ATRIP) in complex with ATR and the Rad9–Rad1–Hus1 (9-1-1) complex to ssDNA [26]. The 9-1-1 complex then recruits DNA topoisomerase-binding protein 1 (TOPBP1) which triggers the ATR-mediated phosphorylation of checkpoint kinase 1 (Chk1) [26]. The Rad17– replication factor C complex, the 9-1-1 complex, and the adaptor protein claspin are also required for Chk1 activation [25,27]. The Rad17–replication factor C complex acts as a clamp loader for the 9-1-1 complex [25] and claspin links ATR and Chk1, allowing for the phosphorylation of Chk1 on serine 317 and serine 345 [28]. Of these phosphorylation sites, serine 345 is essential for Chk1 activation, while serine 317 plays a contributory role [29]. Once active, Chk1 prevents the activation of Cdk1 by phosphorylating Cdc25A and Cdc25C, targeting them for cytoplasmic sequestration by the 14-3-3 proteins [30] or for ubiquitination and degradation by the proteasome [31]. This prevents the removal of inhibitory phosphates on threonine 14 and tyrosine 15 of Cdk1, preventing Cdk1 activity. Active Chk1 also stabilizes the Wee1 kinase, which is responsible for phosphorylating tyrosine 15 of Cdk1 [32]. It has been reported that Chk1, but not Chk2, is essential for the activation of the G2/M checkpoint. In 2000, Liu et al. generated an inducible Chk1 deficient line of murine embryonic stem cells [28]. They found that when this cell line was irradiated and Chk1 depleted, these cells abrogated the G2/M checkpoint [28]. It has also been demonstrated that H1299 human lung carcinoma cells treated with doxorubicin (a What were the first experiments done to know that there is there a cytoplasmic signal that drives the cell signal ? Experiment: Take G1 phase cells : no DNA synthesis occurs here Fuse these G1 cells with S phase cells : make a single cell with 2 nuclei Even the G1 cells now enter S phase Experiment: Take G1 phase cells : no DNA synthesis occurs here Fuse these G1 cells with M phase cells : make a single cell with 2 nuclei Even the G1 cells now enter M phase The G1 Cyclin and the G1 CDK activity The Relationship between Cyclin levels and CDK activity The Cell Cycle has “Stop” and “Go” Signals - Called “Checkpoints” The Cell cycle control system is regulated by both”Internal” and External controls The 3 Important checkpoints are : - G1/S - G2/M - M (spindle checkpoint: metaphase to anaphase) Cyclin D CyclinE Cyclin A Cyclin B