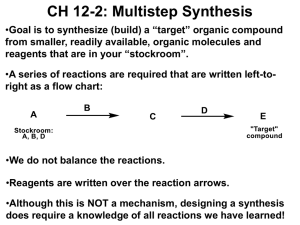
PRACTICE DRAWING ORGANIC MECHANISMS NAME OF STUDENT: NAME OF MARKER: Electrophiles are electron pair acceptors. Nucleophiles are electron pair donors. When starting a mechanism, thinking about the electronegativity of the elements will often get you started. Curly arrows show the movement of a pair of electrons. Curly arrows start at lone pairs, bonds or a ring of delocalised electrons. Curly arrows point to atoms or bonds. Full charges must be shown. Partial charges do not gain marks but may help you work out the mechanism. Incorrect curly arrows lose you marks. 1 Bromoethane and warm aqueous sodium hydroxide Mechanism name and drawing: 2 Equation with reagents, not ions: 3 Name the organic product: 4 Ethanoyl chloride with propan-1-ol Mechanism name and drawing: 5 Equation with reagents, not ions: 6 Name both products: 7 Identify another way to make an ester. Which method is better and explain why? 8 Chemists prefer to use acid anhydrides. Give 2 reasons why. 9 Methylpropene and hydrogen bromide, forming the major product Mechanism name and drawing: 10 Equation with reagents, not ions: 11 Name the organic product: 12 Name the minor product made in the alternative mechanism: 13 Explain why the major product is made. Propan-1-ol with concentrated sulphuric acid 14 Mechanism name and drawing: 15 Equation with reagents, not ions: 16 Name the organic product: Butanone with KCN followed by dilute HCl 17 Mechanism name and drawing: 18 Equation with reagents, not ions: 19 Name the organic product: 20 Does the product have enantiomers? Explain. 21 The product formed does not rotate plane polarised light. Explain. Propane and chlorine to make a secondary halogenoalkane 22 Mechanism name and drawing: 23 Equation with reagents, not ions: 24 Name the organic product: 25 Condition for the reaction: Benzene, AlCl3 and ethanoyl chloride 26 Mechanism name and drawing: 27 Equation with reagents, not ions: 28 Name the organic product: 29 Equations to show the formation of the electrophile and the regeneration of the catalyst: Excess chloroethane and ammonia 30 Mechanism name and drawing: 31 Equation with reagents, not ions: 32 Name the organic product: 33 If an excess of ammonia was used, draw the final product made and state its use 2-bromobutane and hot ethanolic sodium hydroxide 34 Name of mechanism 35 Mechanism drawing 1: 36 Equation with reagents, not ions: 37 Name the organic product: 38 Mechanism drawing 2: 38 Equation with reagents, not ions: 39 Name the organic product: VERY OFTEN IONS TAKE PART IN MECHANISMS BUT THEY ARE NOT REAGENTS – REAGENTS DO NOT HAVE A CHARGE AND YOU CAN HAVE A BOTTLE OF THEM.