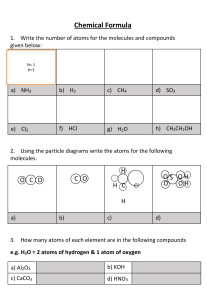
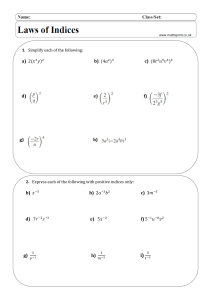
To summarize, the steps for balancing redox reactions in acidic solution are as follows: 1. Divide the reaction into half reactions 2. Balance the elements other than H and O 3. Balance the O atoms by adding H2O 4. Balance the H atoms by adding H+ 5. Balance the charges by adding e6. Add the half reactions and simplify ~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~ To summarize, the steps to balancing a redox reaction in basic solution are as follows: 1. Divide the reaction into half reactions 2. Balance the elements other than H and O 3. Balance the O atoms by adding H2O 4. Balance the H atoms by adding H+ 5. Add OH- ions to BOTH SIDES neutralize any H+ 6. Combine H+ and OH- to make H2O 7. Simplify by cancelling out excess H2O 8. Balance the charges by adding e9. Add the half reactions and simplify