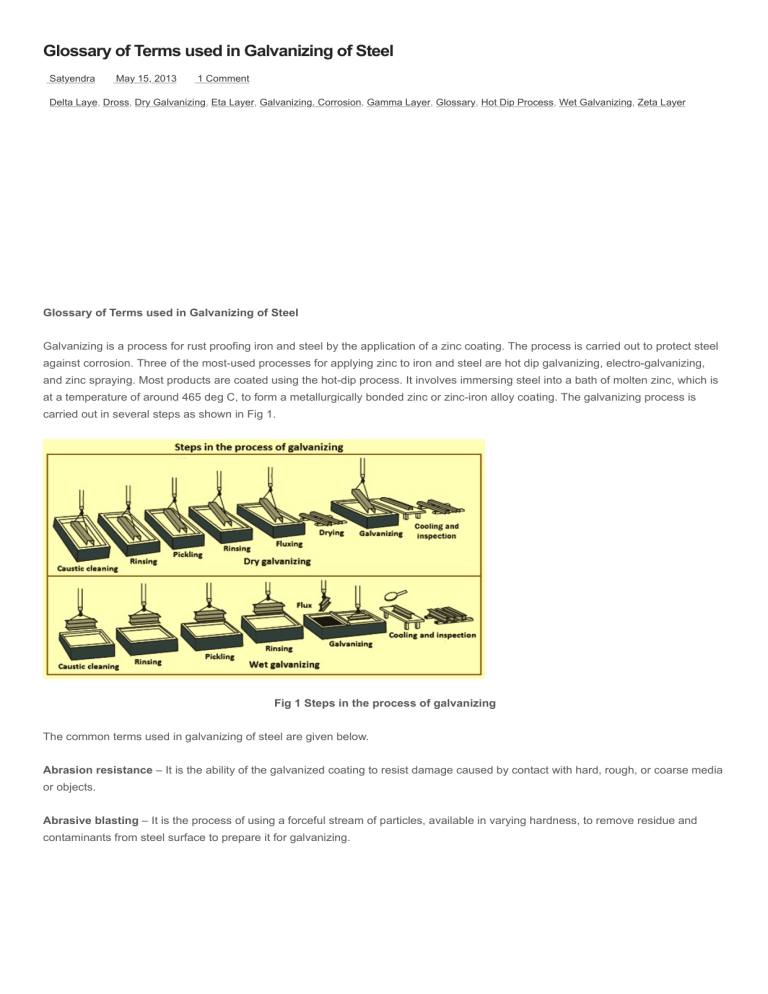
Glossary of Terms used in Galvanizing of Steel Satyendra May 15, 2013 1 Comment Delta Laye, Dross, Dry Galvanizing, Eta Layer, Galvanizing. Corrosion, Gamma Layer, Glossary, Hot Dip Process, Wet Galvanizing, Zeta Layer Glossary of Terms used in Galvanizing of Steel Galvanizing is a process for rust proofing iron and steel by the application of a zinc coating. The process is carried out to protect steel against corrosion. Three of the most-used processes for applying zinc to iron and steel are hot dip galvanizing, electro-galvanizing, and zinc spraying. Most products are coated using the hot-dip process. It involves immersing steel into a bath of molten zinc, which is at a temperature of around 465 deg C, to form a metallurgically bonded zinc or zinc-iron alloy coating. The galvanizing process is carried out in several steps as shown in Fig 1. Fig 1 Steps in the process of galvanizing The common terms used in galvanizing of steel are given below. Abrasion resistance – It is the ability of the galvanized coating to resist damage caused by contact with hard, rough, or coarse media or objects. Abrasive blasting – It is the process of using a forceful stream of particles, available in varying hardness, to remove residue and contaminants from steel surface to prepare it for galvanizing. Acid pickling – It is carried out with normally hydrochloric acid at 10 % concentration for the removal of rust and mill scale from the steel surface prior to galvanizing. Adherence – It is the act, action, or quality of zinc bonding to steel, measured in grams/square meter. Aggressive environment – It is an environment which is particularly corrosive. Air lock – It is an area where air is trapped in a fabrication and prevents the molten zinc from contacting the surface of steel, causing an uncoated area on the work piece. Alloy layers – These are the interior layers of the galvanized coating formed when molten zinc reacts with iron in the steel. The hot dip galvanized coating consists of a series of zinc-iron alloy layers which make up typically 80 % of the coating thickness. These alloy layers are coated with a layer of zinc. The zinc-iron alloys are much harder than zinc with excellent abrasion resistance. The photomicrograph in Fig 2 shows these layers. Fig 2 Photo-micrograph of a galvanized coating Aluminum – It is the element found in the galvanizing bath (added to molten zinc through a product normally called ‘brightener bar’) which gives the hot-dip galvanized coating a shiny appearance. Amphoteric – It is having the characteristics of an acid and a base and capable of reacting chemically either as an acid or a base. Anion – It is a negatively charged ion, especially the ion which migrates to an anode in the electrolysis process. Anode – It is the electrode of an electrolytic cell at which corrosion (oxidation) occurs. Positive current flows from the anode through the electrolyte to the cathode. With respect to hot dip galvanizing, anode refers to zinc, which corrodes sacrificially to protect steel. Anodic – It is the exhibiting of the properties of an anode. Zinc is anodic to steel. Application – It is the act of putting to use; specifically, the use to which galvanized steel is put. Aqueous – It is relating to, similar to, containing, or dissolved in water, or watery. Ash – Ash is the zinc oxidation products formed by the molten zinc reacting with oxygen in the air, and oxidation products arising from the flux reaction form on the surface of the molten zinc. It is the solid by-product consisting primarily of zinc oxides, which remains on the surface of the bath and normally referred to as ‘skimming’. The ash is skimmed off and recycled. Ash inclusions – It is the ash or skimming carried out of the pot on parts. Ash inclusions remain solidified in the coating. Assemblies – It is the fitting together of manufactured parts into a complete structure, machine, or unit of a machine. Assemblies sometimes need specific design features in order to be optimally galvanized. Atmosphere – It is a surrounding influence or environment which affects the rate of corrosion. Frequency and amount of moisture, humidity, chlorides, sulphides, and wind are some of the atmospheric components affecting corrosion rates. Bare spots – These are the defects on the steel surface which have not galvanized because of poor design or poor pre-treatment. Barrier protection – It is the protection provided by inhibiting oxidation (rust) by an insoluble top coating such as zinc, which isolates steel from any electrolytes which assist the corrosion process. Beam work – Beams or head frames are used to support steelwork on wire or hooks to allow it to be handled through the galvanizing process. Blow out(s) – These are the areas adjacent to unsealed overlapping surfaces which have been affected by pre-treatment solutions boiling out of the overlap area. Bond strength – It is the strength with which two or more items are joined. It is the resistance which is to be overcome in order to separate the joined materials, e.g. steel and zinc-iron alloy layers of the galvanized coating, or galvanized reinforcement steel and concrete. Bracing – It is the metal which is attached to a fabrication prior to galvanizing in order to provide support so that the steel does not change shape during heating and cooling. It can be temporary or permanent. Brown staining reaction – It is the reactions between exposed inter-metallic layers (specifically the iron portion of the layers) and oxygen, resulting in surface colour changes from gray to brown. Brush blasting – Galvanized steel needs to be lightly abrasive blasted prior to painting. Brush blasting needed the use of fine abrasive media at relatively low pressure (less than 2.5 atmospheres) to prevent damage to the galvanized coating. Cathode – It is the electrode of an electrolytic cell at which reduction occurs. Positive current flows from the anode (zinc) through the electrolyte to the cathode (steel). Cathodic – It is the exhibiting properties of a cathode. Steel is cathodic in relation to zinc. Cathodic protection – It is the reduction or prevention of corrosion of a metal surface by making it a cathode in an electrolytic cell, using either a galvanic or impressed current. Zinc is higher in the electro-chemical series than iron, and corrodes sacrificially to prevent the corrosion of adjacent exposed steel. Pre-galvanized products (sheet, tube and wire) rely on this feature of the galvanized coating to protect the cut edges of products processed from these sections. Caustic degreasing – All steel products are degreased in a hot caustic solution as the first stage of the pretreatment process for galvanizing. The acid pickling is not effective unless all organic contamination, grease, and oil are removed from the steel’s surface. In the hot dip galvanizing process, organic residues are removed by immersing steel in a tank of caustic solution. Centrifuge work – It is the process of removing excess zinc from small hot dip galvanized parts by placing them in a perforated, rapidly spinning cylindrical container. Small parts which cannot be efficiently handled individually are centrifuged or spun to remove excess zinc and allow them to be processed in bulk in baskets. Nails, washers, bolts, and chains are typical centrifuge products. Chain work – Large or complex steel fabrications which need to be handled individually are suspended on chains for galvanizing. These products include large pipes, box and boat trailers, and heavy items. Chemical cleaning – It is the process of immersing steel in chemical solvents to remove (dissolve) residues which otherwise prevents the galvanized coating from forming. Chromate quenching – It is treating metal in a tank of containing a solution of chromium compounds to produce a conversion coating consisting of trivalent and hexavalent chromium compounds. After galvanizing, the steel item is cooled by quenching in a water bath containing a low concentration of sodium dichromate. The sodium dichromate solution creates a passivation film on the galvanized surface. Chromate passivation sometimes is used on galvanized reinforcement bars to control reactions between zinc and concrete while the concrete cures, particularly the hydrogen evolution which adversely affects bonding. Chromate quenching of galvanized articles prevents the formation of wet storage stain. Chromating – It is the chromate quenching of a galvanized article. Cleaning – It is the process of chemically or mechanically removing unwanted residue or contaminants (mill scale, rust, dirt, and oil) from the surface of a steel article prior to galvanizing. Cleaning solutions – These are liquids used to remove unwanted residue or contaminants (mill scale, rust, dirt, and oil) from the surface of steel prior to galvanizing. Cleaning solution can be typically an alkali, caustic solution, hydrochloric acid or sulphuric acid, and zinc ammonium chloride flux solution. Clearances – Where galvanized components have to fit together (e.g. hinged items, galvanized bolts), sufficient clearance is to be allowed to accommodate the galvanized coating on each surface. Coating mass – Galvanized coatings are generally specified in terms of coating mass, in g/sq. m, on the surface of the steel. For ease of measurement, the thickness of a galvanized coating is measured in micrometers using non-destructive techniques. One micrometer in thickness is around 7 g/sq m in coating mass. Coating thickness – It is the thickness of the zinc coating, measured in micrometers. The hot dip galvanized coating thickness is determined by galvanizing bath chemistry, steel chemistry, steel surface condition, and steel section thickness. National standards normally define minimum acceptable coating thickness for a range of steel sections. Cold galvanizing – It is the process used to touch up and / or repair hot dipped galvanized surfaces and providing barrier protection as well as some cathodic protection. Cold working –It is bending or forming of steel at ambient temperature. This action induces stresses which can be released during the galvanizing process. Containment – It is the act, process, or means to keep the parameters within the prescribed limits. Continuous galvanizing – Sheet, wire, and tube sections are galvanized using a continuous process associated with the manufacturing of the product. The galvanized coating is almost 100 % pure zinc and applied to a maximum thickness of about 30 micrometers. Contraction – It is the shrinkage of steel due to cooling after removal from the galvanizing pot. Corrosion – It the chemical or electro-chemical reaction between a steel and its environment which produces a deterioration of the steel and its properties. Zinc chemically reacts with elements in the atmosphere, thereby sacrificially corroding to prevent corrosion of the underlying steel. Corrosion rate – Galvanized (zinc) coatings oxidize progressively over time. This loss of metal from the surface is deemed to be the corrosion rate and is consistent over time. It is measured in micro-meters/year. A typical corrosion rate for galvanized coatings is 1 micrometers/year to 2 micro-meters/year. Degreasing – It is the first pre-treatment stage in the galvanizing process using a hot caustic soda bath to remove organic contaminants and paint from the steel surface. Delta layer – It is the second layer of zinc iron alloy growth from the base steel formed during the galvanizing process (Fig 2). The chemical composition of the delta layer is around 90 % zinc and 10 % iron. The delta layer is 60 % harder than the base steel it protects from abrasion and corrosion. It is the thickest alloy layer in the galvanized coating. Reactive steels increase the delta layer thickness. Design – It is to create, fashion, execute, or construct steel according to plan so that it yields a quality hot dip galvanized coating. Diamond Pyramid Number – It is system of assigning values to metals quantifying their hardness. Dissimilar metals – These are two or more different metals in contact. Due to varying surface conductivity, one or more metals can experience accelerated corrosion. Since zinc is high in the galvanic series, it preferentially corrodes to protect most dissimilar metals. Dissolution – It is the process of dissolving, splitting, or separating into component parts. Distortion – Some steel sections distort during galvanizing due to differential heating and cooling or inbuilt welding stresses. Distortion is the deviation from the original size, shape or contour which occurs when the application of heat during the galvanizing process releases stress from the steel induced in the fabrication process or during the steel making process. Distortion is of concern when galvanizing asymmetric structural shapes and / or fabrications. Double dipping – It is the process of dipping steel, which is too large in one dimension to completely fit into the galvanizing pot, more than once in cleaning solutions and molten zinc metal in order to produce a coating which covers the entire surface of the steel. Fabricated items longer or wider than the galvanizing bath in one dimension can be galvanized by double dipping, where one side or end of the fabrication is galvanized first. The fabrication is then rotated or turned over allowing the second section to be galvanized. Drainage – It is the act, process, or mode of becoming emptied or freed of cleaning solutions and / or zinc. Fabricated items immersed in molten zinc are to be designed and to allow the zinc to freely drain from internal and external surfaces are to be suspended correctly during the galvanizing process. Dressing – After galvanizing, the coating is inspected and irregularities are removed by dressing the surface by buffing or filing. Dross – Steel reacting with molten zinc forms small zinc-iron crystals in the galvanizing bath. These are heavier than zinc and settle to the bottom of the galvanizing pot where they are periodically removed. Dross is the by-product of the galvanizing process which forms by reactions between zinc and loose particles of iron. Dross can exist at all depths of the pot, but normally sinks to the bottom. Dross inclusions – It is the dross which is carried out on to the work piece upon removal from the galvanizing pot. Drossing – It is the process of removing dross build-up from the bottom of the pot. Dry galvanizing – It is the dipping of steel in an aqueous zinc ammonium chloride solution and then thoroughly drying before immersing in the molten zinc bath. Ductile iron – It is the molten iron treated with an element such as magnesium or cerium to induce a measurable degree of ductility to the iron. These additives do not affect galvanizing ability of iron. Ductility – It is the ability of a material to be formed without fracturing. Galvanized steel is ductile within certain recommended bending radii. Duplex coating system – It is the galvanized steel which has been coated with an additional corrosion inhibiting product, typically liquid, powder, or paint. The two separate coating systems work synergistically to provide enhanced corrosion protection. Duplex coating systems enhance the appearance or durability of the steel being protected.Electrical isolation – It is the separation of two conductive materials from electrical contact. Galvanized steel is sometimes electrically isolated in order to prevent rapid consumption of the zinc coating. Electrode – Anode and cathode are two types of electrode. Electrolyte – It is an ionized chemical substance or mixture, normally liquid which conducts electric currents. Electroplating (Electro-galvanizing) – Zinc is deposited on a clean steel surface from a zinc chemical solution to form a thin, bright zinc coating. Electroplated zinc coatings are not suitable for exterior exposures as they contain very little zinc, typically less than 10 micrometers in thickness. Embrittlement – It is the reduction in the normal ductility of steel due to a physical or chemical change which can occur when cold worked steel is immersed in molten zinc in the galvanizing pot. Some high strength or severely cold-worked steels are susceptible to embrittlement in the galvanizing process. This can be caused by hydrogen embrittlement from the acid pickling, or the heat of the process with severely cold worked (strain aged)) steel. Environment – It is the complex of physical, chemical, and biotic factors (climate, soil, and living things) which act upon metal and ultimately affect the corrosion rate. Eta layer – It is the fourth outer layer of the galvanized coating solely comprised of zinc (Fig 2). Etch priming – Some galvanized coating primers contain acid etching components to improve adhesion. These are application critical products which needs experience in their application. Excess zinc – It is the extra amounts of zinc which can accumulate on the steel because of chemical composition of the steel or the profile of the steel. External venting – These are the holes which prevent high-pressure gas build-up in enclosed fabrications dipped in the molten zinc of the galvanizing bath. Finishing – It is the final stages of inspection and preparation of the galvanized steel so that it complies with the specification. Flux – These are the chemicals used to protect steel from oxidation prior to entering the molten zinc containing pot. Flux inclusions – It is the flux carried out onto the steel from the top flux blanket incorporated in the wet process. It occurs only in the wet galvanizing process. Flux staining – It occurs after galvanizing, when the crevices and overlaps, which are not sealed, can show signs of brown staining bleeding out of the crevices. This is the result of iron-rich flux residues being trapped in the crevices absorbing moisture. Fluxing – Fluxing is the use of a hot zinc ammonium chloride pre-flux solution to condition the cleaned steel prior to its immersion in the molten zinc. It is the process by which steel is dipped in aqueous zinc ammonium chloride to remove undesirable substances and to protect it from further oxide formation prior to entering the galvanizing bath. Galling – It is a condition whereby excessive friction between high spots on two different steel parts results in localized welding. Galvanizing – It is applying of a protective coating of zinc to steel by immersing the cleaned steel in the molten zinc. The zinc and steel react to form the galvanized coating. Galvanizing provides barrier and cathodic protection from corrosion. Galvanizing alloy – Galvanizing baths are alloyed with small amounts of other metals such as aluminum, nickel, or lead to improve the fluidity and resistance to oxidation of the zinc. Galvanizing temperature – It is the temperature at which the molten zinc bath is kept in order to react with the steel. Typically this temperature is between 443 deg C and 454 deg C. Gamma layer – It is the zinc-iron alloy layer closest to the surface of the steel in the galvanized coating (Fig 2). It is the first layer of zinc iron alloy growth from the base steel formed during the galvanizing process. The chemical composition of this layer is around 75 % zinc and 25 % iron. Gamma layer is the hardest layer in the coating and has a diamond pyramid number (DPN) of 250 compared to the DPN of 159 for the base steel. Gray coatings – Some steels produce a matt gray galvanized coating. These coatings are 100 % alloy layer and contain no free zinc. They tend to be thicker than standard shinier galvanized coatings. Grinding – It is the mechanical removal of the material from a work piece with a grinding wheel or abrasive belt. Grit blasting – It is abrasive blasting with small irregular pieces of steel, malleable cast iron, or hard non-metallic materials. Handling – It is the process by which steel articles are carried throughout the galvanizing facility, by chain, wire, hook, or racked in a fixture. Hardness – It is the resistance of metal to plastic deformation, normally by indentation. The term can also refer to the stiffness, or temper, or to resistance to scratching, abrasion or cutting. Holding devices – These are the fixtures used to connect fabrications / parts to be galvanized to handling equipment in the galvanizing facility. Hot rolled steel – It is the steel deformed plastically at such a temperature and strain rate that re- crystallization takes place simultaneously with the deformation thus avoiding strain hardening. This is the most common type of steel galvanized. Hydrochloric acid – It is the solution used in the cleaning stages of the galvanizing process and consisting of one hydrogen ion and one chloride ion (chemical formula: HCl) in mixture with water. Hydrogen embrittlement – It is a condition of low ductility in metals resulting from the absorption of hydrogen. High strength (over 800 MPa) steel is susceptible to hydrogen embrittlement arising from hydrogen in the acid pickling solutions penetrating the steel surface. Jig – A specially designed fixture for holding fabricated items during the galvanizing process to improve quality and productivity. Identification – It is the marking / labeling of steel so that different customer products can be distinguished from one another after galvanizing. Impact resistance – It is the ability to avoid damage due to contact with a forceful motion or object. Galvanized coating’s uppermost, pure zinc Eta layer is relatively soft and absorbs impact shock, protecting the underlying alloy layers. Inspection – It is coating thickness and surface condition verifications. Intermetallics – These are interior layers of the galvanized coating which have distinct proportions of the alloying metals iron and zinc, examples are delta, gamma and zeta layers. Internal stress – It is also known as residual stress. It is the stress present in a steel member or fabrication which is free of external forces or thermal gradients. Internal venting – These are holes on the inside of enclosed fabrications which allow cleaning solutions, zinc, and any gases to freely flow throughout the structure. Kettle – It is the molten zinc filled tank or pot where the metallurgical bonding of zinc and steel takes place. Lifting points – These are connectors (sometimes temporary) directly on the steel work pieces article which help the galvanizer in handling the work piece throughout the galvanizing process. Magnetic thickness test – Non-destructive measurement of galvanized coatings is normally done with electronic instruments which measure the distance from the surface of the coating to the steel surface which is magnetic. Any non-magnetic coating over steel can be measured with these instruments. Masking –This is the process of using a material to produce intentionally ungalvanized areas, typically used in areas which are to be welded, on faying surfaces, or areas where the galvanized coating is not necessary for uniform corrosion protection. Matte – It is dull, lacking or deprived of shine. Matte gray galvanized appearance can result from steel chemistry or can be intentionally induced when the use of the galvanized steel defines reflectivity limits. Mechanical cleaning – It is the removing of residues or impurities from steel using mechanical force such as grinding or sand blasting. Metalizing – Zinc wire or powder is applied to an abrasive blast cleaned steel surface through a gas flame which melts the zinc. Metalizing is forming a metallic coating by atomized spraying with molten zinc or by vacuum deposition. It is also called spray metalizing. It is applying an electrically conductive metallic layer to the surface of another material. Metalizing is used to repair large damaged areas of galvanized coating. Metallurgical bond – It is the bonding of iron / zinc inter-metallic layers to the base steel. Mill lacquer – It is the organic protective coating applied to steel parts, normally pipes or tubes, to protect the parts during shipping. This material cannot be removed by the normal galvanized cleaning methods. Mill scale – It is a heavy, embedded iron oxide layer formed during hot fabrication or heat-treatment of steels. Nickel – It is the common element found in the galvanizing pot to suppress the reactivity of silicon and phosphorus in the steel. Normalizing – It is the heat of the galvanizing process which is insufficient to affect steel properties and which performs a stress relieving (normalizing) function.Organic contaminants – Theses are surface impurities (dirt, grease, oil and paint markings) which hinder the formation of the galvanized coating, normally removed in the caustic cleaning stages of the galvanizing process. Overtapping – It is the cutting of female fastener threads of nuts or threaded holes larger than standard to account for the increased diameter of the galvanized (male) mating part. Passivation –It is changing of a chemically active metal surface to a much less reactive state. Galvanized items are quenched in a weak sodium di-chromate solution to passivate the fresh galvanized surface and allow it time to develop its protective oxide layer. Patina – It is the relatively insoluble zinc carbonate layer which is that formed as the galvanized coating weathers, providing added corrosion protection and abrasion resistance. Phosphating – It is forming of an adherent phosphate coating on steel by immersion in a suitable aqueous phosphate solution, normally used to promote better adhesion of paint to galvanized steel. Phosphorus – It is naturally occurring element normally found in steel, particularly reactive in molten zinc metal. Pickling – It is removing surface oxides from steels by immersion in ambient temperature, dilute hydrochloric acid or hot (82 deg C) sulphuric acid. Pimples – These are small lumpy inclusions which can sometimes occur in galvanized coatings, caused by dross stirred up from the bottom of the galvanizing pot. Pinhole – It is small hole left in a weld area which allows low viscosity liquids to enter and become pressurized under the high temperature conditions of the molten zinc bath. Pitted surfaces – These are the areas of steel where small, sharp cavities exist, normally formed by the corrosion. Polarization – It is the partial or complete polar separation of positive and negative electric charges in a nuclear, atomic, molecule, or chemical system. Post treatment – It is subjecting the steel to specific processes after it has been galvanized. Pre-flux – It is the process of fluxing steel before it enters the galvanizing pot as opposed to using a top flux layer, which is located on top of the molten zinc in the pot. Pre treatment – It is subjecting steel to specific processes before galvanizing. Progressive dipping – It is the act of dipping steel more than once in cleaning solutions and molten zinc metal in order to produce a coating which covers the entire surface of the steel. It is normally done when the steel article / fabrication is too large to fit entirely into the pot in one dip. Quenching – It is rapid cooling by dipping galvanized steel in a tank filled with a liquid solution which is normally water or a dilute chromate or phosphate solution. Racking – It is the process of arranging articles on a rack in order to transport them more efficiently through the galvanizing process. Reactive steel – Some grades of steel react more quickly with molten zinc. This is normally caused by steel chemistry, particularly silicon and phosphorous content. Repair – It is performing finishing work on the piece after galvanizing in order to meet standards or specifications, or coating areas of steel which have been exposed due to post galvanizing fabrication, installation, or extremely rough handling. Residue – These are contaminants (oil, grease, dirt, rust and mill scale) which unless removed, prevents complete galvanizing of the steel surface. Return current path – It is the path through which the current in an electric cell returns to the source. Rinsing – It is removing of any active solution from the surface of steel by immersion in water rust, corrosion product consisting of hydrated iron oxides. Rust – It is the corrosion product consisting of hydrated iron oxides. Rust staining – It is the reaction between exposed inter-metallic layers (specifically the iron portion of the layers) with oxygen, which cause mild red or brown staining. Salt water – It is the water with high concentrations of sodium chloride or other salts. Scale – It is a thick layer of embedded oxidation (rust) products on the steel. Seal welding – It is a weld used primarily to obtain tightness and prevent the flow of cleaning solutions and zinc into otherwise enclosed areas, to prevent flash steaming which causes localized ungalvanized areas. Service life – It is the anticipated length of time when zinc protects steel. It is the amount of time until enough of the galvanized coating is consumed and 5 % of the substrate steel surface area shows signs of rust. Shot-blasting – It is the abrasive blasting of steel with metal shot, normally done to remove deposits or mill scale more rapidly or more effectively than can be done by sand-blasting or chemical cleaning. Silicon – It is naturally occurring element commonly found in steel. Silicon is particularly reactive in the molten zinc metal. Silicon steel – Steel high in silicon give rise to thicker coatings which can be gray in appearance. Skimming – It is a galvanizing byproduct comprised mainly of zinc oxides. The skimming is recyclable. Skip welding – It is alternating the weld so that it is not continuous or complete. Spangle – It is the characteristic crystalline form exhibited by the solidified, hot dipped zinc coating (Fig 3). Crystalline formations on the surface of the galvanized coating are caused by the presence of lead and other alloying elements in the galvanizing bath. Fig 3 Sprangled structure of galvanized steel Stenciling – It is the process by which lettering or a design through which a substance (ink, paint, or metallic powder) is forced onto a surface to be printed. It is normally used to mark steel fabrications but generally does not remain after the galvanizing process. Storage – It is the area where galvanized articles are staged for pick-up or delivery. Strain age embrittlement – It is the loss in ductility accompanied by an increase in hardness and strength which occurs when low carbon steel is aged following plastic deformation. The degree of embrittlement is a function of aging time and temperature, occurring in a matter of minutes at the galvanizing temperature but requiring a few hours to years at room temperature. Steel which has been severely cold-worked by bending of punching is susceptible to strain-age embrittlement. The onset of this type of embrittlement is accelerated by the heat of the galvanizing process. Stress relieving – It is heating to a suitable temperature, holding long enough to reduce residual stresses and then cooling slowly enough to minimize the development of new residual stresses. Structure – It is the steel member of specific cross-sectional dimensions used in fabrication and / or construction. Sulphuric acid – It is a solution used in the cleaning stages of the galvanizing process. It consists of two hydrogen ions and one sulphate ion (chemical formula: H2SO4) in a water mixture. Surface preparation – It is stages of cleaning which prepares the steel for finishing (galvanizing).Tank – It is the container for chemicals used in the galvanizing process. Steel is dipped sequentially in solution containing tanks. Temporary bracing – It is the metal which is attached to a fabrication prior to galvanizing in order to provide added support so that the steel does not change shape during heating and cooling. Temporary bracing is removed after galvanizing. Touch up – It is performing of the finishing work after galvanizing in order to meet standards or specifications, or coating areas of steel which have been exposed due to post galvanizing fabrication, installation, or extremely rough handling. Venting – It is providing holes in fabrications to be galvanized to allow entrapped, heated liquids and gases to escape as pressure increases. All hollow sections are to be vented to allow molten zinc to freely enter and leave the fabrication, and allow condensation or moisture to escape. Vibrating – It is the process of removing excess zinc by rapidly shaking galvanized articles. Weepage – It is the leaching out of trapped liquid solutions in galvanized structures, primarily through pinholes or gaps in welds which are not sealed over by zinc. Weld residue – It is the impurities left from the welding process. Weld residue inhibits localized formation of the galvanized coating. Weld slag – It is the material resulting from the combination of weld material and weld flux. Weld slag inhibits localized formation of the galvanized coating. Wet galvanizing –It is using a liquid flux layer floated on top of the molten zinc. In the galvanizing process, final cleaning occurs as the material passes through the flux blanket before entering the molten zinc bath. Wet storage stain – It is the white surface oxide and hydroxide which forms on newly galvanized steel when excessive moisture is present in poorly ventilated storage. White rust – It is bulky white oxide, sticky substance comprised of basic zinc hydroxide or zinc carbonate. It forms when galvanized surfaces are constantly covered by water or water containing sulphides or chlorides. It can also form on galvanized coatings if they are stored in damp, poorly ventilated conditions. Zeta layer – It is the outer alloy layer in galvanized coating (Fig 2) containing around 94 % zinc and 6 % iron. This layer sometimes merges with the delta layer, depending on the steel chemistry. Zinc – It is the major element found in the galvanizing pot which provides both barrier and cathodic protection for steel. Zinc ammonium chloride – It is the typical component of the flux solution used in the cleaning phase of the galvanizing process. Zinc carbonate patina – It is relatively insoluble zinc carbonate layer which forms as the galvanized coating weathers, providing added corrosion protection and abrasion resistance. Zinc hydroxide – It is corrosion product formed in response to the presence of moisture on galvanized articles. Zinc oxide – It is basic corrosion product formed almost instantaneously on freshly galvanized articles after withdrawal from the molten zinc metal. Zinc patina – It is relatively insoluble zinc carbonate layer which forms as the galvanized coating weathers, providing added corrosion protection and abrasion resistance. Zinc solder – It is the material used to touch-up and / or repair hot dip galvanized surfaces. Zinc iron alloy layers – These are inner layers of the galvanized coating formed from inter diffusion reactions between iron in the base steel and molten zinc metal, (e.g. delta, gamma, and zeta). Zinc rich paint – This is also called cold galvanizing. It is the material used to touch up and or repair hot dipped galvanized surfaces, providing barrier protection and some cathodic protection.

