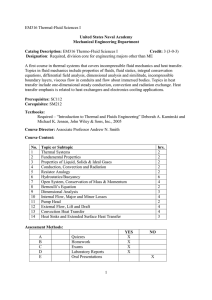
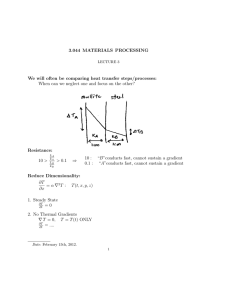
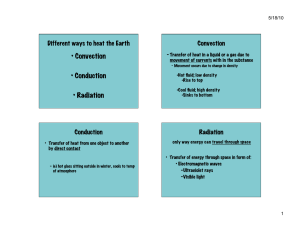
Chemical Engineering The subjects are :I. Thermodynamics II. Fluid Mechanics III. Material Balance and Energy IV. Heat Transfer V. Mass Transfer VI. Chemical Reaction Engineering Now I am going to write a brief summary on all the above subjects. I. Thermodynamics :- This gives the basics of our course Chemical Engineering. Thermodynamics is the study of energy, including the conversion of energy from one form into another and the effects that adding or removing energy have on a system.Thermodynamics is essential for the practice of chemical engineering. The principles of thermodynamics have a fundamental role in how chemical processes are understood, analyzed, and design.Thermodynamics, science of the relationship between heat, work, temperature, and energy. In broad terms, thermodynamics deals with the transfer of energy from one place to another and from one form to another. It talks about the concept of system and surroundings. The boundary layer concept also comes into play .The key concept is that heat is a form of energy corresponding to a definite amount of mechanical work. Thermodynamics has recognized three fundamental laws, simply named by an ordinal identification, the first law, the second law, and the third law. The First Law of Thermodynamics The first law of thermodynamics, also known as Law of Conservation of Energy, states that energy can neither be created nor destroyed; energy can only be transferred or changed from one form to another. For example, turning on a light would seem to produce energy; however, it is electrical energy that is converted. The Second Law of Thermodynamics The second law of thermodynamics says that the entropy of any isolated system always increases. Isolated systems spontaneously evolve towards thermal equilibrium—the state of maximum entropy of the system. More simply put: the entropy of the universe (the ultimate isolated system) only increases and never decreases. The Third Law of Thermodynamics The third law of thermodynamics states that the entropy of a system approaches a constant value as the temperature approaches absolute zero. The entropy of a system at absolute zero is typically zero, and in all cases is determined only by the number of different ground states it has. Specifically, the entropy of a pure crystalline substance (perfect order) at absolute zero temperature is zero. This statement holds true if the perfect crystal has only one state with minimum energy. II. Fluid Mechanics:- Fluid mechanics is the study of fluid behavior (liquids, gases) at rest and in motion. Fluid mechanics has a wide range of applications in chemical engineering. The basic fluid mechanics principles are the continuity equation (i.e. conservation of mass), the momentum principle (or conservation of momentum) and the energy equation. A related principle is the Bernoulli equation which derives from the motion equation Fluid mechanics helps us understand the behavior of fluid under various forces and at different atmospheric conditions, and to select the proper fluid for various applications.Some of the most fundamental concepts of fluid properties are temperature, density, and composition. Mass and volume are examples of extensive properties, which are properties that depend on the amount of material. Density, temperature, and pressure are examples of intensive properties. Application of Fluid Mechanics:1. 2. 3. 4. 5. Hydraulic machines. ... Thermal Power Plants. ... Nuclear power plants. ... Fluids as a Renewable Energy Source. Operating Various Instruments III. Material Balance and Energy:- The material balance is the fundamental tool of chemical engineering. It is the basis for the analysis and design of chemical processes. ... The material balance is the chemical engineer's tool for keeping track of what is entering and leaving the process as well as what goes on internally.A mass balance, also called a material balance, is an application of conservation of mass to the analysis of physical systems. ... By accounting for material entering and leaving a system, mass flows can be identified which might have been unknown, or difficult to measure without this technique. Material balance is based on the mass conservation principle which states that the sum of the weight of all inputs must be exactly equal to the sum of all outputs. ... Second, it helps estimate the conversion or yield of processes which serves as a measure of the efficiency of the process in mass terms. Material and energy balances are very important in an industrial process. Material balances are fundamental to the control of processing, particularly in the control of yields of the products. The first material balances are determined in the exploratory stages of a new process, improved during pilot plant experiments when the process is being planned and tested, checked out when the plant is commissioned and then refined and maintained as a control instrument as production continues. If there is any change in the process, the material balances need to be determined again. The increasing cost of energy has caused the industries to examine means of reducing energy consumption in processing. Energy balances are used in the examination of the various stages of a process, over the whole process and even extending over the total production system from the raw material to the finished product. The law of conservation of mass leads to what is called a mass or a material balance. Mass In = Mass Out + Mass Stored Energy balances can be calculated on the basis of external energy used per kilogram of product, or raw material processed, or on dry solids or some key component. The energy consumed in food production includes direct energy which is fuel and electricity used on the farm, and in transport and in factories, and in storage, selling, etc.; and indirect energy which is used to actually build the machines, to make the packaging, to produce the electricity and the oil and so on. IV. Heat Transfer:- Heat transfer is thermal energy in transit due to a temperature difference.Heat transfer deals with temperature gradients and non-equilibrium phenomena.Heat transfer, any or all of several kinds of phenomena, considered as mechanisms, that convey energy and entropy from one location to another. The specific mechanisms are usually referred to as convection, thermal radiation, and conduction (see thermal conduction).The Three Mechanisms of Heat Transfer: Conduction, Convection, and Radiation.Modes of Heat Transfer:- There are three basic modes of heat transfer; Conduction, Convection and Radiation. 1. Conduction It is a mode which requires a material medium for the transfer of heat. The material medium is called a body and it could be a Solid or a Liquid or a Gas. In Solids, if the temperature of a certain portion of the body is increased by transfer of heat then it means the internal energy of that portion of body has increased. The molecules which are present at that location of body vibrate to and fro and in the process they will interact and collide with neighboring molecules thus transferring energy in the process. The solids also contain free electrons, these free electrons are also responsible for conduction of heat as they move from their location to elsewhere, get diffused and collide with other free electrons. The distance between the molecules in Liquids and Gases is larger as compared to solids. For conduction to happen the activity of molecules in liquids and gases is such that they don’t move very far from their initial positions. Due to the large distances between molecules the extent of conduction is relatively small as compared to solids. The rate of heat conduction through a medium depends on the geometry of the body, temperature differential within the body and the type of material medium itself. 2. Convection This mode also requires a material medium for heat transfer. It results due to the bulk movement of the molecules of the medium from one part of body to the other part due to existence of a temperature difference between those two portions of the body. Convection occurs in Fluids. Whenever convection takes place there is always a solid object or a solid surface in contact with the fluid or immersed in the fluid. The solid object usually transfers heat and causes the fluid to move. When there is no bulk motion in fluid the heat transfer occurs by mode of conduction. For convection to happen there should be an appreciable bulk motion in the fluid. Convection is called Forced Convection if the fluid is forced to flow over the solid or around the solid by external means such as fans, pumps or blowers. Convection is called Natural Convection or Free Convection if the fluid motion is caused by Buoyancy forces that are induced by density differences due to variation of temperature in the fluid. 3. Radiation It is the only mode of heat transfer which does not require a material medium. Radiation is an energy emitted by matter in form electromagnetic waves. Whenever convection takes place there is always a solid object or a solid surface in contact with the fluid or immersed in the fluid. The solid object usually transfers heat and causes the fluid to move. When there is no bulk motion in fluid the heat transfer occurs by mode of conduction. For convection to happen there should be an appreciable bulk motion in the fluid. Convection is called Forced Convection if the fluid is forced to flow over the solid or around the solid by external means such as fans, pumps or blowers. Convection is called Natural Convection or Free Convection if the fluid motion is caused by Buoyancy forces that are induced by density differences due to variation of temperature in the fluid. We also studied about Boiling , Evaporation, Condensation , Heat Exchanger etc. V. Mass Transfer :- This is one of the subjects which is taught only in Chemical Engineering discipline. Mass transfer is the net movement of mass from one location to another or travel of individual chemical species from high-concentration regions to low-concentration regions.Mass transfer describes the transport of mass from one point to another. Mass transfer may take place in a single phase or over phase boundaries in multiphase systems. In the vast majority of engineering problems, mass transfer involves at least one fluid phase (gas or liquid), although it may also be described in solid-phase materials. In many cases, the mass transfer of species takes place together with chemical reactions. This implies that flux of a chemical species does not have to be conserved in a volume element, since chemical species may be produced or consumed in such an element. The chemical reactions are sources or sinks in such flux balances. The theory of mass transfer allows for the computation of mass flux in a system and the distribution of the mass of different species over time and space in such a system, also when chemical reactions are present. The purpose of such computations is to understand, and possibly design or control, such a system. The driving forces for mass transfer are relatively easy to define. These forces give the expressions for the flux, which can be used in the equations for conservation of mass. When these equations are discretized using a numerical method and when the resulting numerical model equations are solved, the results give the concentration distribution and fluxes in a system as functions of the modeled space coordinates and time. The estimates of the concentration and fluxes can be used to understand, design, optimize, and control the system that is being studied. VI. Chemical Reaction Engineering:- Chemical reaction engineering aims at studying and optimizing chemical reactions in order to define the best reactor design. ... Chemical reaction engineering approaches are indeed tailored for the development of new processes and the improvement of existing technologies. This subject covers the knowledge of reaction kinetics, reactor design and separation which distinguisheschemicalengineerfromotherengineers.•Thecourseintroducesthebasicreactordesigncalculationan ddesignofcommercialchemicalreactors,emphasizingsynthesisofchemicalkineticsandtransportphenomena.•T hetopicscoverinthissubjectarekineticratetheory,homogeneousreactioninbatchandflowsystems,heterogeneous reactionandcatalysis,temperatureeffect,effectofheattransferandcatalyticreactoralsoreactordesign,sizingandm odelingofperformance. CREdealswithchemicallyreactivesystemsofengineeringsignificance.•Chemicalreactionengineeringisthediscip linethatquantifiestheinteractionsoftransportphenomenaandreactionkineticsinrelatingreactorperformancetoope ratingconditionsandfeedvariables.•CREisneededinthedevelopmentofnewandtheimprovementofexistingtechn ologies.–searchforalternativeprocessestoreplaceoldones•novelreactors(useofmetallocenecatalysts)– findroutestomakeaproductfromdifferentfeedstock•novelprocessesforsynthesisgasproduction•Hydrocarbonproductionfromsyngas•Biodieselproduction– reduce/eliminateunwantedbyproducts•fuel-cellsforautomobiles•NOxreduction CRE is perhaps the key course that differentiates ChemicalEngineering & other Engineering Thus this is the brief summary of all the core subjects we have studied till date.