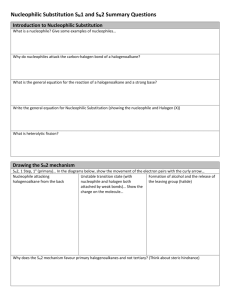
Halogenoalkanes (Chapter 16) Note: This is a final version of the notes for chapter 16. There will not be any further version of notes for this, and this will be used for the final exam as preparation material Halogenoalkanes: alkanes where one or more hydrogen atoms have to be replaced by a halogen atom CH3CH3 → CH3CH2Cl [chloroethane] [undergoes free radical substitution mechanism] When naming a halogenoalkane: - If it has a chlorine atom, the prefix should be chloro- If it has a fluorine atom, the prefix should be fluoro- If it has a bromine atom, the prefix should be bromo- It if has a iodine atom, the prefix should be iodio- Isomers of C5H10Cl2: [1,1-dichloropentane] [1,3-dichloropentane] [2 optical isomers] [2,3-dichloropentane] [4 optical isomers] [1,2-dichloropentane] [1,4-dichloropentane] [2 optical isomers] [2,4-dichloropentane] [4 optical isomers] [2 optical isomers] [1,5-dichloropentane] [1,1-dichloro 3-methylbutane] Elimination Reactions: Reagents: halogenoalkane, ethanolic NaOH Conditions: Heat CH3CH2Cl + NaOH → CH2=CH2 + NaCl + H2O [one H and Cl atom is removed] Note: Ethanolic NaOH is NaOH dissolved in ethanol. It is not a aqueous solution [2-chloropropane] [2-chlorobutane] [prop-1-ene] [cis but-2-ene] [but-1-ene] [prop-1-ene] [trans but-2-ene] Hydrolysis of Halogenoalkanes: [making alcohol] Using aqueous OH-ions: Reagents: Halogenoalkane, aqueous NaOH Conditions: Heat under reflux CH3CH2Cl + NaOH → CH3CH2OH + NaCl [chloroethane] [ethanol] Using Water: Reagents: Halogenoalkane, water Conditions: Heat under reflux CH3CH2Cl + H2O → CH3CH2OH + HCl [ethanol] Note: using OH- ions results in a much faster reaction Rates of Hydrolysis of Halogenoalkanes If acidified silver nitrate is added to the hydrolysis reaction mixture, then precipitate is formed - A white precipitate is formed if a chloroalkane is being hydrolysed - A cream precipitate is formed if a bromoalkane is being hydrolysed - A yellow precipitate is formed if a iodoalkane is being hydrolysed The rate of formation of precipitate will be the rate of hydrolysis Note: if you have a halogenoalkane but you don’t know which halogen it has, a hydrolysis reaction can be carried out while adding a acidified silver nitrate The faster the hydrolysis takes place, the faster the halogen ion is produced, hence, faster the precipitate is produced ● ● ● ● Iodoalkanes hydrolyse the fastest, because the C-I bond is the weakest Bromoalkanes hydrolyse the second fastest, because the C-Br bond is stronger Chloroalkanes hydrolyse the third fastest, because the C-Cl bond is more stronger Fluoroalkanes hydrolyse the slowest, because the C-F bond is the strongest C-I > C-Br > C-Cl > C-F [fastest] [slowest] Substitution with Cyanide (CN-) ions: Reagents: halogenoalkanes, ethanolic KCN Conditions: Heat under reflux CH3CH2Cl + KCN → CH3CH2CN + KCl [propionitrile] Note: this reaction is used to increase the number of carbon atoms Uses of CH3CH2CN: 1. Can be reduced to form amines → CH3CH2CH2NH2 [propylamine] [CN becomes CH2NH2] [it is a reduction reaction] Reagents: propanenitrile Sodium and ethanol are used to carry out the reaction 2. Hydrolysis of nitriles → CH3CH2COOH [propanoic acid] CH3CH2CN + H2O + H+ → CH3CH2COOH + NH4+ Aqueous H+ ions and heat used Substitution with Ammonia: Reagents: halogenoalkane, ethanol ammonia Conditions: heat, high pressure in a sealed container CH3CH2Cl + NH3 → CH3CH2NH2 + HCl [ethyl amine] Primary, Secondary and Tertiary Halogenoalkanes primary halogeno alkane because the alpha carbon atoms is attached to only 1 other carbon atom secondary halogenoalkane because the alpha carbon atom is attached to two different carbon atoms tertiary halogenoalkane because the alpha carbon atom is attached to three different carbon atoms The Nucleophilic Substitution Mechanism Sn2 Primary halogenoalkanes only undergo Sn2 mechanisms Sn2: - S stands for substitution n stands for nucleophilic 2 means two reacting species involved [halogenoalkane] [nucleophile] Steps of Nucleophilic Substitution Mechanism Sn2: 1. The Cl atom in the C-Cl bond tries to pull the pair of electrons towards itself 2. The nucleophile (electron rich) attacks the C-Cl bond 3. This results in an intermediate stage where a half bond is created 4. The Cl atom pulls the pair of electrons and ethanol is formed The Nucleophilic Substitution Mechanism Sn1 Tertiary halogenoalkanes only undergo Sn1 mechanisms Sn1: 1 means two reacting species involved [halogenoalkane] Steps of Nucleophilic Substitution Mechanism Sn1: 1. Heterolytic fission occurs, where the Cl atoms takes the bonding pair of electrons 2. A tertiary carbocation forms, which is then attacked by a nucleophile 3. Butanol is formed Difference between Sn2 and Sn1: ● Sn2 is direct attack of nucleophile on partial positive carbon atom, then an intermediate stage occurs, and the final product then forms ● Sn1 carbocation forms first, nucleophile attacks the carbocation, then we get the final product Note: secondary halogenoalkanes can undergo either Sn2 or Sn1 Why do primary halogenoalkanes only undergo Sn2? Because a primary carbocation is formed. They are less stable than tertiary carbocations A few more examples: Uses of Halogenoalkanes: - Propellants in aerosols - Refrigerants in fridges - Anaesthetics - Used in Pipes (PVC) Used in non-stick pans (Teflon) Problems associated with halogenoalkanes: 1. The ozone layer in the atmosphere protects us from the harmful radiation coming from the sum 2. Chlorofluorocarbons (CFCs) cause the ozone layer to break down a. This is due to the homolytic fission of the Cl-Cl bond caused by UV light b. This results in formation of chlorine radicals c. These radicals react with the ozone layer Solutions for CFC’s: 1. HFC’s (hydrofluorocarbons) - aerosols, propellants, fridges 2. HFE’s (hydrofluoroethers) - solvents, cleaning and drying agents