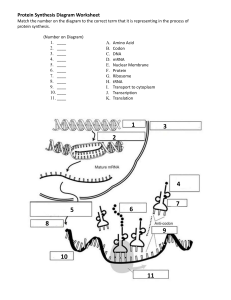
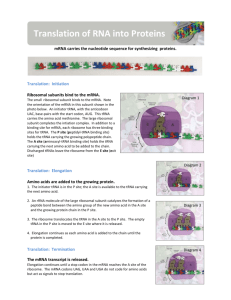
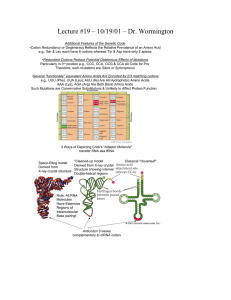
Protein Translation Molecular Components Process Description Initaition Small ribosomal subunit binds to mRNA and initiator t-RNA (met). Attachment of Large Ribosomal subunit completes the translation initiation complex. Proteins called initiation factors bring all components together. One molecule of GTP is expended by cell to bring all components together. mRNA tRNA, GTP Ribosomes initiator tRNA-met initiation factors Elongation rRNA Elongation Factors GTP x 2 Termination Release Factor GTP x 2 Chapereronin Post Process Modification/ Other Events SRP- Signal Recognition Protein Aminoacyl-tRNA Synthetase, ATP tRNA, animo acids Prokaryotes vs. Eukaryotes Anticodon of tRNA basepairs with complementary mRNA at codon in A site of the Large Ribosome, with the hydrolysis of one GTP. rRNA molecule of Large Ribosomal subunit catalyzes formation of a peptide bond. Ribosome translocates tRNA in A site to P site, and uncharged tRNA from P site to the E site, which gets released. One molecule of GTP is expended. Elongation Factors are required, and it is the mRNA that moves. Stop Codon in mRNA reaches A site in Ribosome. Release factor binds to stop in A site with addition of a water molecule to break the bond between completed polypeptide and tRNA in P site and releasing protein through exit tunnel of Large Ribosomal subunit. Translation machinery breaks down with the expenditure of two GTP molecules. The polypeptide chain begins to coil and fold spontaneously as a consequence of its amino acid sequence forming a protein with a specific shape: a three-dimensional molecule with secondary and tertiary structure. Certain amino acids may be chemically modified by the attachment of sugars, lipids, phosphate groups, etc. Enzymes may remove one or more amino acids from the leading (amino) end of the polypeptide chain. In some cases, a polypeptide chain may be enzymatically cleaved into two or more pieces. SRP recognizes signal peptide (20 aa) at amino end of protein while synthesis is happening and transports protein to ER or Golgi if protein is destined for an endomembrane system or secretion out of cell. The translocation complex in the membrane cleaves the signal peptide and holds the protein and signal. Protein is released to the ER lumen or stays partially embedded in the ER membrane. tRNA is used repeatedly Aminoacyl-tRNA Synthetase binds amino acid and ATP molecule, appropriate tRNA covalently binds to amino acid displacing AMP (3’end tRNA) In Prokaryotes, the small ribosomal subunit can bind mRNA and tRNA in either order; it binds the mRNA at a specific RNA sequence, just upstream of the start codon, AUG. In Eukaryotes, the small ribosomal subunit, with the initiator tRNA already bound, binds to the 5' cap of the mRNA and then moves, or scans, downstream along the mRNA until it reaches the start codon, and the initiator tRNA hydrogen bonds to itestablishes codon reading frame of mRNA. Large Ribosomal subunit binds which completes the translation initiation complex. Bacteria can simultaneously transcribe and translate the same gene because it has no compartmental organization. Eukaryotes segregate transcription from translation and provides a compartment for extensive RNA processing. This processing stage includes additional steps whose regulation can help coordinate the eukaryotic cell's elaborate activities. 1