Physical Science Practice Test: Kinetic Theory, States of Matter
advertisement

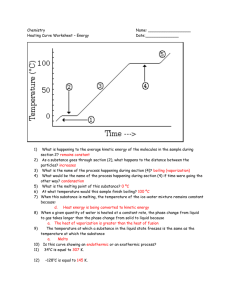
Physical Science Period: Name: Date: Practice Test for Unit 3: Ch. 3, and some of 15 and 16: Kinetic Theory of Matter, States of matter, and energy and thermodynamics, and gas laws. 1. The Kinetic Theory of Matter states that all matter is composed of are in a constant state of that 2. What is Brownian motion, and why does it happen in terms of kinetic theory? 3. What is thermal expansion, and why does it happen in terms of kinetic theory? 4. What is diffusion, and why does it happen in terms of kinetic theory? 5. According to the Kinetic Theory what does temperature measure? (don't say "heat") 6. How does a thermometer work? 7. Why can nothing be cooled to below absolute zero? 8. How does the freezing point of a substance compare to the melting point of the same substance in the same atmospheric conditions? Why is that? 9. Consider the properties of solids, liquids, and gases we have covered in class. a) Fill in the following table for the the common states of matter in terms of relative intermolecular bond strength (small, medium, or large), particle movement (vibrating only, flowing fast, or flowing slowly), particle organization (random or orderly), particle spacing (in contact or not in contact), and type of shape and volume each has (variable or definite). State of Relative bond Particle Particle Particle Type of Type of shape mater strength movement organization spacing volume Solid Liquid Gas b) Explain using the kinetic theory of matter. 10. Draw the phase change triangle, listing the 3 common phases of matter, naming the transitions between them, if the changes are endothermic or exothermic changes and why that makes since. 11. Consider evaporation and boiling. a) What are two main differences between evaporation and boiling? b) How is it similar to boiling? 12. What is the first law of thermodynamics, and what would that limit about a bouncy ball that is dropped from a certain height? Why is that? 13. What is the second law of thermodynamics, and what would that limit about a bouncy ball that is dropped from a certain height? Why is that? 14. All forms of energy can be divided into one of two main categories of energies, what are those two main energy types. Give at least three examples of forms of energy in each of the two types. 15. What happens to the temperature of a substance during a phase change? Why is that? 16. Draw a sketch of the temperature vs. energy graph for ice at -20ºC being heated to steam at 120ºC. Label where the H2O is 1) a solid, liquid, or gas, 2) taking in or releasing energy, 3) going through a phase change and what change that is called. 17. What causes gas pressure (not what affects it, but what causes it in the first place)? 18. What happens to the pressure of a gas in a closed container if the volume decreases, but temperature remains the same? Why? 19. What happens to the volume of a gas in a closed container if the temperature increases, but the pressure remains the same? Why? 20. What happens to the temperature of a gas in a closed container if the pressure decreases but the volume stays the same? Why? 21. What happens to the volume of a gas in a closed container if the pressure decreases but the temperature remains the same? Why? 22. What happens to the pressure of a gas in a closed container if the speed of the gas increases, but the volume and number of gas particles remains the same? Why? 23. What happens to the pressure of a gas in a closed container if the original gas was replaced by a new gas that has more massive gas particles, but maintains the same number and speed of gas particles as the original? Why? 24. Consider 0°C water and the same mass of 0°C ice a) What has more internal energy, the water or the ice? Why is that? b) What has more average kinetic energy the water or the ice? Why is that? c) What has more stored energy, the water or the ice? Why is that? 25. How if at all would your answers to question 24 change if we were considering 100°C water and 100°C steam instead of ice and water. 26. Which is usually larger, the amount of energy to turn a solid into a liquid at the m.p., or the amount of energy to turn the same amount (in terms of mass) of a liquid into a gas at the b.p.? Why is that? 27. Below is Temperature vs. Time graph of a certain substance being heated and then cooled. Assume the same amount of energy is added or removed each second. 160 140 120 Temperature (degrees C) 100 80 60 40 20 0 0 100 200 300 400 500 600 700 800 900 1000 -20 -40 -60 time (s) a) On the graph, label where, if anywhere, the substance is: 1) solid, liquid, or gas; 2) melting, freezing, vaporizing, or condensing; 3) taking in energy or releasing energy b) What are the melting, boiling, and freezing temperature of the substance. How do you know? i. Melting point = , because: ii. Freezing point = , because: iii. Boiling point = , because: c) At what time was the substance removed from the heat? know? d) What took a longer time, the object to melt or the object to vaporize? Assuming the same amount of energy is added each second, why is that? How do you 28. Use the information below to answer questions A - H Substance Melting Point Boiling Point Water 0 100 Iron 1535 2750 Mercury -38.8 356 Lead 327 1751 Ammonia -78 -33 Ethyl acetylene -126 8 a) What temperature scale is being used here? How do you know? b) At what temperature would ammonia change from a gas to a liquid? How do you know? c) In what phase is iron at a temperature of 2600 °C? How do you know? d) At what temperature (in Kelvin) will lead melt? How do you know? e) Is it possible to boil mercury in a lead container? Why? f) What, if anything, happens to iron when the temperature drops from 1800o to 1500? Why is that? g) At what temperature does lead freeze? How do you know? h) Which of the substances would be a solid, which would be a liquid, and which would be a gas in a standard refrigerator (temp of about 3.3 °C)? How do you know? 29. Circle the two temperatures below that are not possible? 7,000,000 K - 25 K -400 °F -279 °C 5,000,000 °C 30. Why are those temperatures circled in #29 not possible? 31. Why is it much better to understand a process or concept than to memorize different aspects?