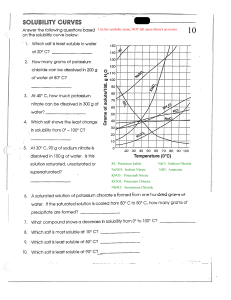
Word Equations Worksheet Write the word equations for each of the following chemical reactions: 1) When dissolved beryllium chloride reacts with dissolved silver nitrate in water, aqueous beryllium nitrate and silver chloride powder are made. 2) When isopropanol (C3H8O) burns in oxygen, carbon dioxide, water, and heat are produced. 3) When dissolved sodium hydroxide reacts with sulfuric acid (H2SO4), aqueous sodium sulfate, water, and heat are formed. 4) When fluorine gas is put into contact with calcium metal at high temperatures, calcium fluoride powder is created in an exothermic reaction. 5) When sodium metal reacts with iron (II) chloride, iron metal and sodium chloride are formed. For chemistry help, visit www.chemfiesta.com © 2002 Cavalcade Publishing, All Rights Reserved Word Equations Worksheet - Solutions Write the word equations for each of the following chemical reactions: 1) When dissolved beryllium chloride reacts with dissolved silver nitrate in water, aqueous beryllium nitrate and silver chloride powder are made. BeCl2(aq) + 2 AgNO3(aq) Æ Be(NO3)2(aq) + 2 AgCl(s) 2) When isopropanol (C3H8O) burns in oxygen, carbon dioxide, water, and heat are produced. 2 C3H8O(l) + 9 O2(g) Æ 6 CO2(g) + 8 H2O(g) 3) When dissolved sodium hydroxide reacts with sulfuric acid, aqueous sodium sulfate, water, and heat are formed. 2 NaOH(aq) + H2SO4(l) Æ Na2SO4 + 2 H2O(l) 4) ∆H = negative ∆H = negative When fluorine gas is put into contact with calcium metal at high temperatures, calcium fluoride powder is created in an exothermic reaction. ∆ F2(g) + Ca(s) Æ CaF2(s) ∆H = negative 5) When sodium metal reacts with iron (II) chloride, iron metal and sodium chloride are formed. 2 Na(s) + FeCl2(s) Æ 2 NaCl(s) + Fe(s) For chemistry help, visit www.chemfiesta.com © 2002 Cavalcade Publishing, All Rights Reserved