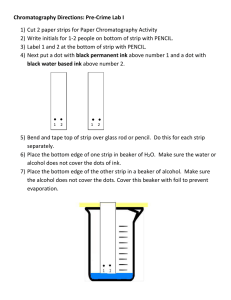
Lab #1: Paper Chromatography To separate a mixture using paper chromatography. Vocabulary Words: • Chromatography _______________________________________ • Homogeneous Mixture __________________________________ • Stationary Phase _______________________________________ • Mobile Phase __________________________________________ • Analyte _______________________________________________ • Solvent _______________________________________________ • Constituent ____________________________________________ There are 3 primary colors: Red, Blue & Yellow. Most colors are produced by mixing two or more primary colors together. Ex. Green color is produced by mixing blue & yellow. In paper chromatography, small samples of analytes are spotted onto chromatography paper, which serves as stationary phase. A liquid mobile phase is wicked up the stationary phase by capillary action, causing the analytes to move upward. The distance traveled by each analyte depends on its affinity for the mobile phase. The higher the affinity the farther it will travel. On the other hand, if the particular analyte has a higher affinity for the stationary phase than to the mobile phase, then it will stick to the stationary phase and not travel as far. The ration of the distance traveled by an ink “spot” (analyte) to the distance traveled by the solvent (mobile phase) is called retention factor, Rf. $*%+(,(! -. /&%,.$( Rf = !"#$%&'( !"#$%&'( $*%+(,(! -. 01,+(&$ • • • • • • • • Black ink Filter Paper Metric Ruler Clear Tape Pencil Isopropyl Alcohol DI Water 250 ml Beaker • • Timer Camera to Record 1. Obtain Filter Paper and 250 ml Beaker 2. Measure and cut 12 cm of Filter Paper 3. Measure 1 cm from the base of the Filter Paper and make a straight line across using a pencil. x x x x pg. 1 Lab #1: Paper Chromatography 4. Obtain a pencil and a tape. Attach the filter paper to the pencil such that when the pencil is rested on the rim of the beaker, the filter paper barely touches the bottom of the beaker. x x x x x x x x 5. Add 5 mL of isopropyl alcohol to 250 mL of beaker. 6. Use the assigned 4 black markers to make the spots on the filter paper. (Warning: The ink should be at least 3 mm from the edge of the filter paper, since liquids travel faster on the edges of a paper. Can you think of why placing ink on the edge of the paper will cause an experimental error and unsuccessful separation of colors). x x x x 7. REST the pencil-filter paper setup on the rim of the beaker with isopropyl alcohol. 5 mL of Isopropyl Alcohol x x x x 8. Observe and Record ! pg. 2 Lab #1: Paper Chromatography Data Table 1: Assigned Black Inks Black Ink Assigned Black Ink # Black Ink Information 1. 2. 3. 4. Data Table 2: Observations Black Ink Assigned Black Ink # Observations in 1 min. Observations in 5 min. Observations in 15 min. Observations in 25 min. 1. 2. 3. 4. (Keep in mind that a few variations of the experimental procedure might be observed). Example #1. (Note: did not use pencil) Example #2. (Note: Then only used 3 Different Black Inks) pg. 3 Lab #1: Paper Chromatography (Note: You must calculate retention factor for each primary color of each black ink). Use the following example to complete your calculations for each of the black inks assigned to your group: $*%+(,(! -. /&%,.$( Rf = !"#$%&'( !"#$%&'( $*%+(,(! -. 01,+(&$ 1) Black Ink # _____ Distance that Mobile Phase Traveled (Solvent) 6 cm 5 cm Distance Blue Dye Traveled Distance Pink Dye Traveled 2 cm 0 cm Original position of the black ink Rf (BlueDye) = 2.4 '5. 6.4 '5 = 0.83 Rf (PinkDye) = 7.4 '5. 6.4 '5 = 0.33 Rf (YellowDye) = 8 6.4 '5 = Repeat this Calculation for all 4 Black Ink Results. 2) Black Ink # ______ 3) Black Ink # ______ 4) Black Ink # ______ Mandatory Watch: Khan Academy Calculating Retention Factor for T.L.C. pg. 4 Lab #1: Paper Chromatography Answer questions # 1-13 1. Restate and clarify the purpose of this lab in your own words including “what will be analyzed, the technique that will be used and what is it that we were trying to accomplish. 1-2 sentences. 2. What is analyte? (definition in your own words) 3. Which substance/substances are considered analyte(s) in this lab? (Temperature, beaker, filter paper etc.) 4. How many analytes did you use in this lab? 5. Identify this(these) analyte(s). 6. In your own words, write the summery of the process of paper chromatography. (How did you conduct this experiment?) 7. Identify each item of the lab as stationary phase, mobile phase, solvent and analyte. 8. Was your experiment successful? 9. Identify possible source of errors. a. ___________________________ b. ___________________________ c. ___________________________ 10. Report your results (your final observation for each analyte). Analyte Name or Analyte Number 1. 2. 3. 4. pg. 5 Lab #1: Paper Chromatography 11. Report all the values of Rf and explain what they mean in terms of degree affinity towards stationary or mobile phase. (use your knowledge of paper chromatography technique. See the background info provided for this lab). Black Ink # Dye Color Rf Values Explain 1. 2. 3. 4. 12. Explain why Rf does not have units such as cm, m, km, etc. (Hint: the answer lies in Rf formula) 13. Do all blue dyes separated from the 4 analytes tested have the same Rf values? What does this tell you about the molecular shapes of those blue dyes? 14. Are these analytes consider to be Homogeneous or Heterogeneous mixtures? Explain. pg. 6