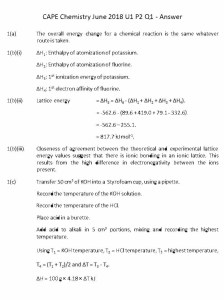
Pyp reflection 9701/41/o/n/21 1. The reaction is spontaneous when both enthalpy and entropy is positive, and the temperature is high. Value for enthalpy is usually given in kJmol-1 so when calculating for delta G it must be multiplied by 1000. 2. the pH of both solutions means individually, the ques doesn't say that the solutions are mixed together. pKa is -log Ka, use Ka = [H+][A-]/ [HA]. Buffer solution may exceed its capacity when all ions have reacted with the H+, so there is no more ions to remove the excess H+ and pH can't be maintained. 3. Electron affinity is the enthalpy change when 1 mole of electrons is added to 1 mole of gaseous atoms or ions to form 1 mole of gaseous negative ions under standard conditions. The first EA is always negative as there is an attraction between the (-) electron and (+) nucleus, so energy is released during bond formation and the enthalpy becomes negative. The second EA is always positive as there will be repulsion between negative charges, so energy is needed to overcome it. Lattice energy depends on charge density. When charge is greater, there are stronger forces of attraction between the oppositely charged ions and more energy is needed for bond formation. 4. Smaller ionic radius will have greater polarizing power. BaCO3 requires higher temperature to decompose, as it is larger and they do not polarize the (CO3)2- ions as much, so the C-O bonds are not distorted and less weakened. Going down the group, solubility of hydroxides increases while solubility of sulfates decreases. BaSO4 is therefore less soluble than CaSO4, because enthalpy of solution is more endothermic, and the change in enthalpy of hydration is less than the change in lattice energy. 5. Recalled ligand exchange reactions and observations. Bidentate ligand is a species that donates two lone pairs of electrons to form two dative bonds to a central metal ion. 6. CuSO4 is coloured because of d-d orbital splitting. The d-orbitals are split into two energy levels, and the electron at lower energy level becomes excited and jumps to a higher energy level by absorbing the wavelength of light. Complementary colour to the wavelength absorbed is then seen. CuI, on the other hand, has a complete d orbital, so no electron will jump to a higher energy level and no complementary colour will be seen. A more negative electrode potential value means that the one on the bottom right is a strong reducing agent, while the one on the top left is a strong oxidising agent. Decreasing the concentration of Cu+ shifts the position of the equilibrium to the right (to form more Cu+) hence increasing the electrode potential value. 7. Acidity of phenylethanoic acid > phenol > ethanol. In phenylethanoic acid, electrons are drawn towards the C=O bond, making the COO- delocalised and weakening the OH bond, so H+ is easily released. In phenol, lone pair of electrons in O overlaps with the pi bonding system of the benzene ring and gets delocalised to form phenoxide ion which is stable and won't react with H+. Ethanol has an electron-donating group so ethoxide ion becomes more negative and easily reacts with H+. Use KCN (ethanolic, heated) to form one additional carbon. Hydrolysis of nitrile to COOH requires dilute HCl and heat. 8. Amine does not react with aqueous bromine but phenylamine does. It decolourises bromine and produces a white solid. Amine group will activate positions 2,4,6. 9. PCl5 in rt / PCl3 + heat / SOCl2 can be used to prepare acyl chlorides. PCl5 is most popular as SOCl2 produces two poisonous gases. Reduction of amide to amine requires LiAlH4 in dry ether, while reduction of nitrile to amine requires LiAlH4 or H2/Ni catalyst. NH and OH peaks will disappear if D2O is added. 10. Isoelectric point is the pH at which it exists as a zwitterion. Zwitterion is when NH2 exists as NH3+ and COOH exists as COO-. In acidic conditions, NH3+ & COOH. In basic conditions, NH2 & COO- 9709/42/o/n/21 1. Lattice energy is affected by ionic radius and charge. A greater charge will result in a more exothermic lattice energy, while a greater ionic radius will result in a less exothermic lattice energy. For NaCl and RaS, the dominant factor is ionic charge, so RaS has a more exothermic lattice energy. 2. Value for Kpc may be less as the mixture is analysed immediately and not allowed to reach equilibrium. Common ion effect shifts the position of equilibrium to the left, hence producing more PbI2(s), and a yellow ppt is seen. 3. Quantitative electrolysis uses Q=ne and Q=It. When temperature is increased for a reaction with + enthalpy and - entropy, -TS becomes more positive, so G becomes more positive and the reaction becomes less spontaneous. 4. Ion polarization is affected by ionic radius. Larger ions will have less polarizing power, so Sr(NO3)2 is more thermally stable than Mg(NO3)2. Going down the group, solubility of hydroxides increases. Mg(OH)2 is less soluble, and Sr(OH)2 is more soluble. Lattice energy decreases more compared to the enthalpy of hydration, so enthalpy of solution becomes more negative. Thus, Sr(OH)2 is more soluble. 5. Recalled ligand exchange reactions and colour changes. A change in colour is due to changes in the d-orbital splitting. Molybdenum complex is Mo(CO)6, iron(III) complex is [Fe(CN)6]3-.[Cu(NH3)4(H2O)2]2+ +4Cl- —> [CuCl4]2- + 4NH3 +2H2O. 6. Kstab = [product]/ [reactant][reactant]^mole. A positive Kstab value means that the product is more stable than the reactant. A positive electrode potential value means that it is a feasible forward reaction. To oxidise sth, must find the one that is more easily reduced than it. So Ecell must be more + 7. For benzene 1,4-dicarboxylic acid, two symmetrical lines can be drawn jd kyk + sign, so carbon environment for that region is the same. COOH is an electron withdrawing group so it directs position 3. The reagent for the oxidation of compound Q (COOH- C6H4-CH3CO) to COOH-C6H4-COOH is alkaline KMnO4 which is then acidified and boiled. Alkane: free radical substitution. Alkene: electrophilic addition. OH/C-X: nucleophilic substitution. Aldehyde/ketone; nucleophilic addition. Benzene: electrophilic substitution. 8. Mixture of amino acids can be maintained at a certain pH with a buffer solution during electrophoresis. Charge and relative molecular mass affects the distance travelled by an amino acid. An amino acid with a greater charge and smaller Mr will travel further. Electrophoresis diagram should include dc power supply, filter paper soaked in buffer sol, mixture of amino acids, glass slide. 9. Recalled different ways and reagents to prepare amine. Halogenoalkane + NH3 —> amine + HCl, reagent; excess NH3 under pressure. Nitrile + [H] —> amine, reagent: H2 with Ni catalyst. Amide + [H] —> amine + water, reagent: LiAlH4 dry ether. Basicity depends on the availability of the lone pair of electrons to accept H+. Butylamine has an e- donating alkyl group, making N more ready to bond with a H+. Phenylamine is weaker as the lone pair of electrons in it overlaps with the benzene ring and becomes delocalised. 10. Reaction of phenol with Na and NaOH, Diazotisation reaction, reaction of phenol with COClC6H4COCl. Ethanol does not react with NaOH or diazonium. Phenol + COClC6H4COCl happens at rt but if ethanol need conc.H2SO4. 9701/41/m/j/21 1. Going down group 2, hydroxides become more thermally stable as cations increase in ionic radius and polarizing power decreases, making the bonds less distorted and weakened. Hence, more energy is needed to break the bonds. Solubility of Ca(OH)2 in NaOH(aq) decreases due to common ion effect of OH- which shifts the equilibrium to the left. More Ca(OH)2(s) is formed so Ksp (solibility) decreases. 2. A complex ion might be non-polar bc dipole moments cancel each other out 9701/42/m/j/21 1. Complex ion is a molecule or ion formed by a central metal ion. Ligand exchange reaction is used when the reaction is not a precipitation or acid-base reaction. Colour of complexes can be different due to difference in energy gap between d-orbitals because of different frequency of light absorbed. 2. pH + pOH = 14. Calculating moles must look at what is reacting and if everything is used up. 3. Standard electrode potential is the voltage of a half-cell compared to a hydrogen halfcell at 1 moldm-3, 1 atm and 298 K. Electrons flow and is released from anode to cathode. The electrolyte on the anode usually has a more negative E value, while the one on the cathode has a more positive E value. Isomer of octahedral ligands are usually classified as optical isomerism. Ksp and Kc only includes elements in aq form. Higher Kstab value means higher concentration. 4. Lattice energy is the enthalpy change needed to form one mole of ionic compound 4. from its gaseous ions under standard conditions. Latt = -sol + hyd. Greater hyd value is due to smaller ionic radius so stronger attraction to water molecules. Mol of e- = Q / F. Entropy is a measure of disorder of a system. When water is evaporating, Gibbs is 0 when T = 100°C 5. Basicity of ethylamine > phenylamine > 4-nitrophenylamine. Basicity depends on the availability of the lone pair to form a dative bond with H+. Ethylamine is e- donating, making N more negative and reactive. The rest has benzene ring so electrons are delocalised and less ready for bonding. NO2 is e-withdrawing so extra delocalisation. 6. When naming compounds, alphabetical order instead of numerical order. To prepare acyl chloride: PCl5 rt, SOCl2 rt, PCl3 heat 7. Order of reaction is the power to which the concentration of a reactant is raised to in the rate equation. Benzenediazonium ion + water will give phenol. Using conc/time graph to determine rate eq. is getting the gradient. Half life decreases for zero order reaction 8. Rate of hydrolysis (decomposition) of acyl chlorides (RC=OCl) fastest > alkyl chloride (RCl) > Aryl chloride (C6H5Cl) slowest. O in acyl chlorides are e- withdrawing so the C=O bond becomes delocalised and C-Cl bond is weakened. Meanwhile, alkyl chlorides are e-donating so Cl becomes more - and strong, reacting with H+. Aryl chlorides are difficult to break as the lone pairs are delocalised into the benzene ring.