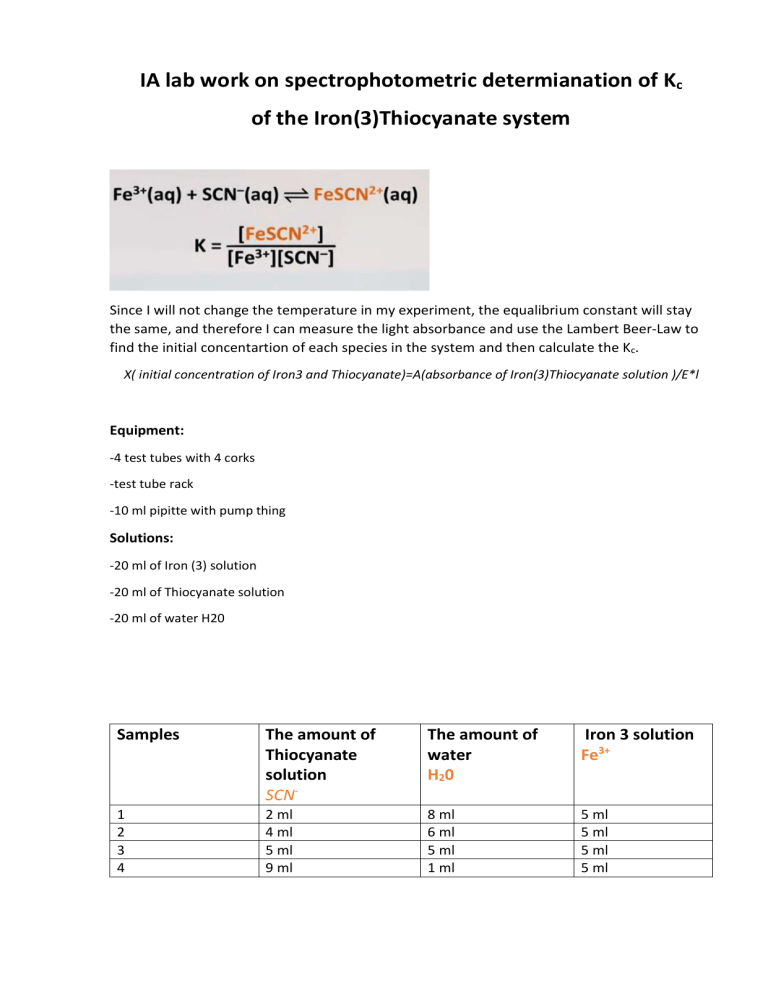
IA lab work on spectrophotometric determianation of Kc of the Iron(3)Thiocyanate system Since I will not change the temperature in my experiment, the equalibrium constant will stay the same, and therefore I can measure the light absorbance and use the Lambert Beer-Law to find the initial concentartion of each species in the system and then calculate the Kc. X( initial concentration of Iron3 and Thiocyanate)=A(absorbance of Iron(3)Thiocyanate solution )/E*l Equipment: -4 test tubes with 4 corks -test tube rack -10 ml pipitte with pump thing Solutions: -20 ml of Iron (3) solution -20 ml of Thiocyanate solution -20 ml of water H20 Samples The amount of Thiocyanate solution SCN- The amount of water H20 Iron 3 solution Fe3+ 1 2 3 4 2 ml 4 ml 5 ml 9 ml 8 ml 6 ml 5 ml 1 ml 5 ml 5 ml 5 ml 5 ml Sample 1st trial (in 2nd trial (in 3rd trial (in 4th trial (in 5th trial nanometers of the molar absorptivity) nanometers of the molar absorptivity) nanometers of the molar absorptivity) (in nanometers of the molar absorptivity) nanometers of the molar absorptivity) 1 (2 ml of Thiocyanate solution) 2 (4 ml of Thiocyanate solution) 3 (6 ml of Thiocyanate solution) 4 (10 ml of Thiocyanate solution) Procedure: 1) 2) 3) 4) 5) 6) Label the test tubes with numbers: 1,2,3,4. Using a pipette, add 5 ml of Iron(3) solution to each test tube. For sample 1, add corresponding amount of water to the 1st test tube. For sample 1, add corresponding amount of Thiocyanate solution to the 1st test tube. Mix it and leave it for 3-4 minutes to reach the equalibrium. Transfer the prepared solution from the 1st tube to the the special small test tube from the spectometer. 7) Put in into spectometer and record the absorbence of light. 8) Repeat step 1-6 with another samples. 9) Do 3 trials for each sample.





