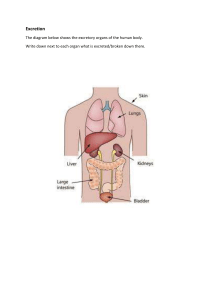
Toxicology I ستار جابـر.د Lecture 3 Toxicokinetics of Xenobiotics Toxicants exert their effect only when they interact with specific receptor sites, which may be located in distant organs. To reach the target site, the toxicant must be absorbed effectively into the blood stream, distributed efficiently to the site of action, and subsequently metabolized and excreted from the body. The onset, duration, and intensity of a substance’s toxic effects depend on the toxicant’s ability to permeate lipid cell membranes directly and its interactions with transporter proteins. Translocations may take place either by simple diffusion or filtration or through specialized transport, which may be active, facilitated, or pinocytosis. Phagocytosis and pinocytosis are proposed mechanisms for cell membranes by which cells engulf 1 small drops of external medium (particles). This type of transfer has been shown to be important for the removal of particulate matter from the alveoli by phagocytes and from blood by the reticuloendothelial system of the liver and spleen. The primary routes of exposure for toxic substances are oral, respiratory, and dermal. The gastrointestinal (GI), respiratory, and dermal systems are lined with epithelia that present significant barriers to the entry of foreign substances due to tight junctions between their cells or continuous lipid layers in the case of skin. The membranes of cells that form viable epithelial barriers are traversed by transporter proteins that either actively exclude xenobiotics or facilitate the movement of specific substrates across the barrier. The plasma membranes surrounding all these cells are remarkably similar. The structure of the plasma membranes thus suggest that the important physico-chemical properties of any xenobiotic that influence its passage across the membrane are its size, shape, degree of ionization, and lipid solubility of the ionized and nonionized forms. Dermal penetration is unique in the sense that the outer epithelial cellular layers (corneocytes) are nonviable and do not contain transporter proteins. Absorption, in this case, is therefore dependent on the ability of toxicants to penetrate the intercellular lipid matrix found between corneocytes. Absorption Absorption is defined as the process of movement of an unchanged compound from its site of administration or exposure to the blood stream. The absorption of xenobiotics is dependent on the solubility of the chemicals, the concentration of chemicals, the circulation, the site of absorption, the area of absorbing surface, and the route of administration. 2 Gastrointestinal Absorption Most of the chemicals are absorbed through the GI tract by the nonionic diffusion across the lipid layer of the GI epithelial membrane. Certain inorganic and organic ions, sugars, amino acids, and pyrimidines are transported by specialized active processes. The chemicals that are weak acids are predominantly ionized at gastric juice pH, poorly absorbed through gastric mucosa, and are absorbed mainly through the intestinal mucosa. If the gastric contents are made alkaline, then acid compounds become more ionized and are less well absorbed. Conversely, basic compounds become less ionized and are better absorbed. In an intestine where the pH is approximately 5.3, the bases are more readily absorbed and weak acids are less readily absorbed than in the stomach. The presence of food can impair the absorption of chemicals; for example, prolonged fasting has been shown to diminish the absorption of several chemicals, possibly by deleterious effects on the epithelium of the intestinal wall. Dermal Absorption Skin is the most common tissue to come in contact with many chemicals. The epidermis, although only approximately 0.2 mm thick, largely limits absorption. some chemicals can be absorbed through the skin in sufficient quantities to produce systemic effects. For example, carbon tetrachloride and other organic solvents penetrate the skin in this way and can cause serious toxic effects. Pesticides (parathion, malathion) and nicotine insecticides have caused deaths in agricultural workers as a result of percutaneous absorption. Chlorovinylarsine dichloride (Lewisite), a mustard gas, is readily absorbed through contact with the skin. The rate and degree of penetration of xenobiotics through the skin can vary in different areas of the body. Because the stratum corneum plays a critical role in determining the cutaneous permeability, abrasion or removal of this layer causes an abrupt increase in the permeability of the epidermis for all kinds of molecules, whether large, small, lipid-soluble, or water-soluble. Several injurious chemicals such as acids, alkalis, or mustard gases can injure the barrier cells, thereby increasing permeability. 3 Dimethylsulfoxide can also facilitate the penetration of toxicants through the skin. This liquid is miscible with water and with many organic solvents, and is capable of increasing the penetration of ionized chemicals into the deeper layers of the skin. Pulmonary Absorption Many xenobiotics inhaled as gases or aerosols enter the circulation by diffusing across the alveolar membranes. Especially, chemicals that have relatively high lipid/ water partition coefficients. Then absorption and excretion of toxic gases and vapors depend primarily on aqueous solubilities (blood/partition coefficients) The chemicals that cause poisoning/disease include asphyxiants and irritants or particulate material, either from the general environment or from occupational disease, such as silicosis. Here, the particle size strongly influences the rate at which material is absorbed through the alveolar epithelium, probably because of the greatly increased surface area for solubilization as the particle diameter becomes smaller. For example, particles of uranium dioxide larger than 3 μ had no toxic effect when introduced into the trachea in rats; however, when smaller particles were introduced, they were absorbed into the circulation and caused kidney damage. If the particle size is larger than 10 μ, then impaction occurs in the pharynx or in the nasal cavity, thus preventing the particle from reaching the alveolar region. Particles between 1 and 5 μ often become sediment within the bronchiolar region of the lung, and particles less than 1 μ can diffuse within the alveoli. The particulates may also be removed via the bronchi and then move to the GI tract. This route is dependent on the number of recruitable macrophages and on the circulation of the fluid surface film lining the alveoli. This route is very important, and approximately 80% of the lung contents of a chemical are cleared by this process. The lymphatic route is another route for the removal of toxicants from the alveoli. Both free and phagocytized particles can penetrate the interstitial tissue of the lung and migrate via the lymphatic system. Some of the particles may remain in the alveolus and form an alveolar dust plaque or module. 4 Distribution Distribution may be defined as a process by which xenobiotics move throughout the body and reach their site of action (extracellular fluid and tissues). Once absorbed, a toxicant typically enters the interstitial fluid at the site of absorption and then passes into the tissue cells or enters the blood and/or lymph. Blood is moved rapidly through the body by the cardiovascular circulatory system, and this process constitutes the major mechanism whereby absorbed chemicals are distributed to the various organs and tissues of the body. Factors that determine a compound’s rate and extent of distribution therefore include: 1. Molecular size (ie, physicochemical properties of compound) 2. Lipophilicity 3. Plasma protein and tissue binding 4. Blood flow and organ size 5. Special compartmental and barriers (eg, blood_brain barrier, blood_cerebrospinal barrier, placental barrier, and other barriers) 6. Availability of special transport system 7. The ability to interact with transmembrane transporter proteins 8. Disease state Excretion Excretion is one of the primary mechanisms of protecting the body from the toxic effects of toxicants through the elimination of these compounds from the body. Elimination encompasses all the processes that decrease the amount of parent compound in the body, including biotransformation. Excretion by principal organs is called renal excretion; excretion by organs other than the kidneys is known as extrarenal or nonrenal excretion. Compounds that are rapidly eliminated are less likely to accumulate in tissues and damage critical cells. The liver can remove the compounds from the blood and prevent their distribution to other parts of the body. Because the liver is the site where the metabolism of most of the compounds occurs, the compound and the metabolites may be excreted directly into the bile without re-entering the blood stream to be excreted by the renal system. A chemical may be excreted by liver cells into the bile and thus passes into small intestine. If the properties of the chemicals or their metabolites are favorable for the 5 intestinal reabsorption, then a cycle may result (enterohepatic cycle) in which biliary secretion and intestinal reabsorption continue until renal excretion finally eliminates the compounds from the body. The biliary route of excretion plays a major role in the elimination of anions, cations, and nonionized molecules containing both polar and lipophilic groups. The bromosulfalein (BSP) has long been used as a test of liver function. The removal of BSP is decreased after liver injury and returns to normal as liver function is restored. Many volatile compounds that enter the body are excreted unchanged in the expired air. For example, fluorobenzene, carbon monoxide, as well as certain alcohols and other volatile agents are excreted by this route. These are all probably eliminated by simple diffusion, and their elimination depends on blood gas solubility. Gases with low blood/gas solubility such as benzene and nitrous oxide are excreted at rapid rate, whereas the chemicals that have higher blood/gas solubility such as ethanol bromobenzene or are excreted very slowly by the expired air. Certain foreign compounds and their metabolites are partly excreted in the milk of lactating mammals. The compounds are excreted in the milk by simple diffusion because the milk is more acidic (pH 6.5- 6.7) than plasma; basic compounds may be present in greater concentrations but the reverse may occur for acidic compounds. Several compounds, such as DDT, thiouracil, tetracycline, and erythromycin, have been reported to be excreted in milk. End Dr. Sattar Jabir 2021 6