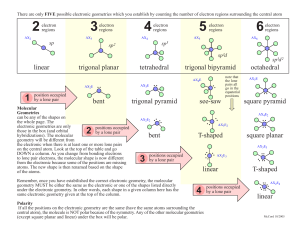
Molecular Geometries and Bonding Theories HS-PS1-1 Molecular Shapes • We’ve learned to draw Lewis structures and account for all the valence electrons in a molecule. • But… Lewis structures are two dimensional and molecules are 3 dimensional objects. • The 3D structure is absolutely critical for understanding molecules. Constructing a Lewis-Dot diagram for molecules Take a screenshot of this Follow these simple steps: 1. Determine total valence electrons for all atoms~ watch out for negative/positive ions (ex: NH41+ 5+4 = 9 -1 = 8 ) 2. Position first atom in the center ~ usually first in the formula (unless H or F). The central atom is supposed to have the lowest electronegativity, meaning it is the farthest to the left on the periodic table 3. Join other atoms with line bonds to the central. 4. Subtract 2 electrons for every bond formed. 5. Place remaining e- around atoms to fill valence. Fill surrounding atoms BEFORE the central atom. 6. Check that each atom has a complete octet. 7. Too few electrons? Give the central atom a complete octet by creating a double or triple bond. Create multiple bonds by moving an electron pair from a surrounding atom into a line bond with the central Your Turn… Use the steps outlined earlier to draw the lewis diagrams for the following molecules 1. PCl3 2. SO3 Molecular Geometry • Lewis structures do not necessarily illustrate molecular geometry, but they do generate the data needed to predict molecular structure. • In the valence shell of the central atom of a molecule are either bonding electron pairs or nonbonding pairs of electrons. These electrons repel creating bond and lone pair angles. VSEPR Valence Shell Electron Pair Repulsion Theory 1. Shared and unshared pairs repel. 2. Lone pairs repel stronger than shared pairs. 3. Double and triple bonds are viewed as single; for structure only. 4. The resultant shape of the molecule is a result of shared and lone pairs being as far apart as possible. What Determines the Shape of a Molecule? • Atoms and lone pairs take up space and prefer to be as far from each other as possible • Shape can be predicted from simple geometry lone pair bonds “Things” • The central atom has four “things” around it. A “thing” is an atom or a lone pair of electrons. • # things = atoms + lone pairs Valence Shell Electron Pair Repulsion Theory (VSEPR) “The best arrangement of a given number of things is the one that minimizes the repulsions among them.” # of things Arrangement Geometry Bond angles Geometries These are the geometries for two through six things around a central atom. You must learn these! Geometries • All one must do is count the number of “things” in the Lewis structure. • The geometry will be that which corresponds to that number of “things.” Molecular Geometries • The geometry is often not the shape of the molecule, however. • The “shape” is defined by the positions of only the atoms in the molecules, not the lone pairs. Geometries vs. Shape Within each geometry, there might be more than one shape. Linear Geometry 2 Things Things Geometry Atoms Lone pairs Shape Example • In this geometry, there is only one molecular geometry: linear. • NOTE: If there are only two atoms in the molecule, the molecule will be linear no matter what the geometry is. Trigonal Planar Geometry 3 Things things geometry atoms lone pairs shape • There are two molecular geometries: • Trigonal planar, if there are no lone pairs • Bent, if there is a lone pair. example Lone Pairs and Bond Angle • Lone pairs are physically larger than atoms. • Therefore, their repulsions are greater; this tends to decrease bond angles in a molecule. Multiple Bonds and Bond Angles • Double and triple bonds place greater electron density on one side of the central atom than do single bonds. • Therefore, they also affect bond angles. Tetrahedral Geometry 4 Things Things geometry atoms lone pairs There are three molecular geometries: • Tetrahedral, if no lone pairs • Trigonal pyramidal if one is a lone pair • Bent if there are two lone pairs shape example Trigonal Bipyramidal Geometry 5 Things Things geometry atoms lone pairs shape example There are four distinct molecular geometries in this domain: • Trigonal bipyramidal • Seesaw • T-shaped • Linear Octahedral Geometry 6 things Things geometry atoms lone pairs shape • All positions are equivalent in the octahedral domain. • There are three molecular geometries example Complete “VSEPR and Polarity” Assignment and submit to Schoology