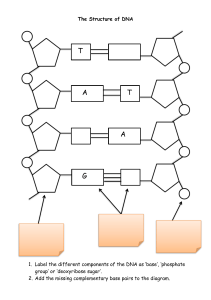
Experiment 5.1: DNA - The Genetic Record Introduction In recent years, forensic investigations have relied increasingly upon DNA evidence both to convict and exonerate suspects. DNA, the genetic ticker-tape” of life, provide often compelling information about crimes and suspects. In order to understand how DNA is employed in forensic investigations, an understanding of some of the basics about DNA itself is first necessary. In 1985, former Marine Kirk Bloodsworth was convicted and sentenced to death for the rape and murder of a nine year old Dawn Hamilton. He received a second trial after a successful appeal based on the grounds of evidence having been withheld at his first trial. However, he was sentenced again, this time for two consecutive life terms. After several years of fighting for a DNA test, samples from the scene of the crime were sent to a lab for testing. The final reports concluded that the DNA found at the scene of the crime did not match Bloodsworth's DNA, and he was released and pardoned by the Governor of Maryland shortly thereafter. Bloodsworth's ordeal lasted 9 years, including two years on death row. The DNA did match that of Kimberly Shay Ruffner, and on May 20, 2004, he pleaded guilty to the crime for which Bloodsworth was convicted. Objectives The purpose of this laboratory experiment is to extract and isolate several samples of DNA from commonly available substances. Additionally, a simple qualitative test will be performed to confirm the presence of DNA in the sample. Background The discovery of DNA is attributed to Johann Miescher (1844 – 1895), a chemist from Switzerland, while working on pus cells. Prior to his work, it was believed that cells were made up largely of protein, long chains of linked amino acid molecules, but Miescher found that certain extracts from cells “cannot belong among any of the protein substances known hitherto”. He successfully showed that these fractions were not protein because they were not digested by protease enzymes (enzymes that break up proteins). These particular extracts were also shown to arise from cell nuclei and were, therefore, named nuclein. Later, work by Albrecht Kossel on nuclein showed that the material contained four nucleic acid bases, and was subsequently renamed a nucleic acid by Richard Altmann. The structure we recognize today as the famous double helical arrangement was explained in 1953 by James Watson and Francis Crick. DNA Extraction and Isolation 1 Copyright James T. Spencer 2004 HO OH CH2 OH CH2 O O C H H C C H H C H C C H C C H H OH Ribose phosphoric acid residue (PO42-), a fivecarbon sugar unit (possible sugars shown in Figure 1), and a nitrogen-containing base (Figure 2) – together these are called a nucleotide. The phosphate group and the deoxyribose sugar, when linked together in an alternating fashion, form a straight chain backbone for the DNA polymer. Off of this backbone are then fastened the nitrogen bases. A complete nuclei acid unit is shown in Fig. 3. Thus, all the units of the backbone are identical (alternating NH2 O -1 P O N N O -1 N CH2 N O C H H H C C OH H C H Figure 3. Nuclei Acid Unit a nuclear DNA polymer, millions and millions of these basic units are strung together. These long strands of DNA polymer are found in nature in complementary pairs, meaning that two are required to form the observed DNA structure. But these strands don’t just come together in any random fashion, but are instead very specific in their interactions. Specifically, two nitrogen bases can come together to form a close electrostatic interaction, called a hydrogen bond, based upon their chemical structure. Thus, cytosine and DNA Extraction and Isolation H OH OH Figure 1. Deoxyribose O DNA is made up, at its most fundamental level, of repeating units of nucleic acids arrayed in a polymeric fashion. Thus, one nucleic acid unit is linked to another, much the way railroad cars are linked together, to form a very long biopolymer. These fundamental nucleic acid units are each composed of three basic parts: a HO NH2 N O N N NH2 N N N N N N NH2 N O O O H3C N N N O N O Figure 2. Nitrogen bases used in DNA and RNA (clockwise from upper left): adenine, guanine, cytosine, thymine (DNA only) and uracil (RNA only). phosphate and sugar) while the pendant nitrogen bases can be different. In fact, DNA uses only four different nitrogen base units: adenine, guanine, cytosine and thymine (RNA, a relative of DNA, replaces thymine with uracil). These bases are shown in Fig. 2. The entire fundamental structure of DNA, therefore, consists of the phosphate-sugar backbone with nitrogen bases hanging off this backbone from the sugar subunit, Figure 4. In a typical strand of O O O O O O H O C C P H O C C P O C C P O C H2 H O C H2 O H O C H2 H H C H O H H H C C N N H O H H C H C N N N NH2 H C N N N N NH2 N N NH2 N Figure 4. Structure of DNA polymer. 2 Figure 5. DNA transcription process. DNA double helix is first opened up in the region containing the genetic information that is to be converted into chemical compounds such as proteins. A complimentary strand is then generated (matching A’s with T’s and G’s with C’s – see below). The new strand (messenger RNA or mRNA) is transferred to a ribosome in the cytoplasm. Each group of three nitrogen bases in the messenger RNA strand designates a specific amino acid compound that is placed in order by a transfer RNA unit (tRNA). The order of the bases in DNA is ultimately transferred into a very specific sequence of amino acids that are linked together to form a new polymer. The polymer formed by linking the amino acids together is called a protein. Protein composition, structure and function (including that of enzymes, structural proteins, and many others) DNA Extraction and Isolation guanine are built to allow a close interaction – close enough to form a hydrogen bond holding the two bases together. Likewise adenine and thymine can also form a hydrogen bonded unit. Having a complementary sequence on the two DNA strands where a cytosine is opposite every guanine and an adenine is opposite every thymine yields a double strand effectively “pinned together” through the nitrogen bases. This is shown schematically in Figure 5. When these complementary strands of DNA come together, they form the wellknow double helical structure (like a twisted flight of stairs). The sequence of the nitrogen bases is the basis of controlling all cellular processes and ultimately determining things like what color eyes we have or how tall we might become. Our genes, the genetic information encoded in DNA, are really composed of just four letters (adenine, guanine, cytosine and thymine) that write all the chemical information required to regulate cellular function. The way that this translates from letters into chemical reactions requires a process called transcription. In a very brief summary, it works something like this. The Figure 6. Hydrogen bonded bases pairs between two strands of DNA. 3 is, therefore, completely dictated by the DNA base orders. As mentioned previously, DNA is found in the nuclei of cells and also in the cell’s mitochondria (called mitochondrial DNA, mtDNA). In the nucleus, it forms the familiar chromosomes that carry all the genetic information. The very long chain DNA molecule in the chromosomes are coiled up tightly to for the chromosomal structures observed as shown in Figure 7. Our DNA, as it turns out, contains far more “data” than is contained within the gene portions of the DNA. The DNA information that makes up a gene, the part that regulates protein sequences, is separated from other genes by regions of DNA that contain essentially nonsense codes (hypervariable region) – codes that do not translate into proteins. These portions may either have had a function in the past but are now not Figure 7. DNA coiling to form chromosomal structures. necessary for cellular function or may have arisen from other means such as mutation or viral insertion. Since protein structure and function derives directly from the ordering of the nitrogen bases in DNA, very small changes in the base ordering in genetic regions of DNA may cause catastrophic changes in cellular function. One base unit incorrectly placed can mean the difference between disease or no disease or even life versus death. For example, the substitution of a single nitrogen base for the correct base in the gene regulating red blood cells may result in a person having sickle cell anemia. Within a species such as humans, there is very little variation of the DNA code within the genetic portions. Mutations, changes in the order of the nitrogen bases in the genetic region of the DNA chain, are not tolerated and usually do not result in viable offspring. Changes (mutations) in the “nonsense” hypervariable DNA regions between the genes, however, usually make no difference in the survival of an organism since these DNA codes play no role in regulating cells or in developing the traits we observe in an organism. Since there is no survival advantage of one code versus another in the hypervariable region, over time these nonsensical regions have become extremely diverse such that essentially no two people have the same DNA codes in these inter-gene regions. The basis, therefore, of forensic DNA is to look at the DNA codes in these inter-gene regions (since looking at the gene regions would not be able to discriminate one persons DNA from another – we’re essentially all the same in the gene regions). In forensic applications of DNA, one of the first tasks is to isolate the DNA from samples gathered at a crime scene. In this laboratory experiment, we will isolate DNA samples from two different sources, one plant and one animal. We will then confirm the presence of DNA in the samples through a chemical qualitative analytical test. DNA Extraction and Isolation 4 DNA: The Genetic Record Experimental Methods I. DNA Extraction: In this experiment, you will extract DNA from a biological sample. Record your measurements and observations on the data sheets provided. Procedure: I.A. Preparation of DNA buffer solution. To make the DNA buffer solution, you will need to measure out 2.5 mL shampoo (without conditioner) or 1.5 mL liquid dishwashing detergent. To this, add 3.75 g NaCl and 25 mL of water. Mix this solution very gently, but thoroughly, so as to avoid forming soapy bubbles as much as possible. This solution is the DNA buffer solution. I.B. Extraction Strawberry DNA. Place one strawberry in a zip lock baggie and smash the strawberry vigorously for about two minutes. To this, add 10 mL of the DNA buffer solution (made in I.A. above) to the bag and reseal. Smash the contents to completely mix then for about one minute. Filter the mixture from the baggie through cheesecloth into a beaker (plastic cup) and then pour the filtrate (the solution in the beaker) into test tube so that it is 1/8 full. Very slowly pour cold alcohol (either 95% ethanol or 95% isopropyl alcohol that has been cooled for at least 15 min. in an ice bath) into the tube so as to form two layers until the tube is about one-half full. At the interface of the layers, you will see the DNA precipitate out of solution and float. Spool the DNA onto a glass rod or pipet tip and collect. DNA Extraction and Isolation 5 DNA: The Genetic Record Data Sheet Name Instructor Laboratory Section Lab Period Step Observation IA IB - strawberries IIA - strawberries DNA Extraction and Isolation 6 DNA: The Genetic Record Post-lab Assignment Name Instructor Laboratory Section Lab Period (1) Suppose a student added the ethyl alcohol straight to the solution from the baggie WITHOUT filtering it through the cheesecloth first. What do you think the student would observe? Would the student be successful in extracting the DNA? Explain briefly why you think this. (2) Would you expect similar results if you were to use other cells, such as liver, onion, or yeast? Briefly explain your reasoning. (3) Consider a single celled organism, such as a bacterium, whose DNA is not enclosed in a membrane-bound nucleus: a. Would you predict that it would be easier or harder to extract the DNA from the bacterium compared to the extraction of DNA from the strawberry or cheek cell? b. Would the single cell organism have as much DNA as the multi-celled organism examined in this experiment? Briefly explain. DNA Extraction and Isolation 7 DNA: The Genetic Record Pre-lab Assignment Name Instructor Laboratory Section Lab Period (1) Using a MSDS sheet, can be found online, describe the specific hazards associated with the following reagents: a. Ethyl alcohol b. Diphenylamine Reagent (2) Why do we perform the following steps: a. Addition of the buffer solution to the baggie b. Filtering the solution through cheesecloth c. Adding the ethyl alcohol DNA Extraction and Isolation 8